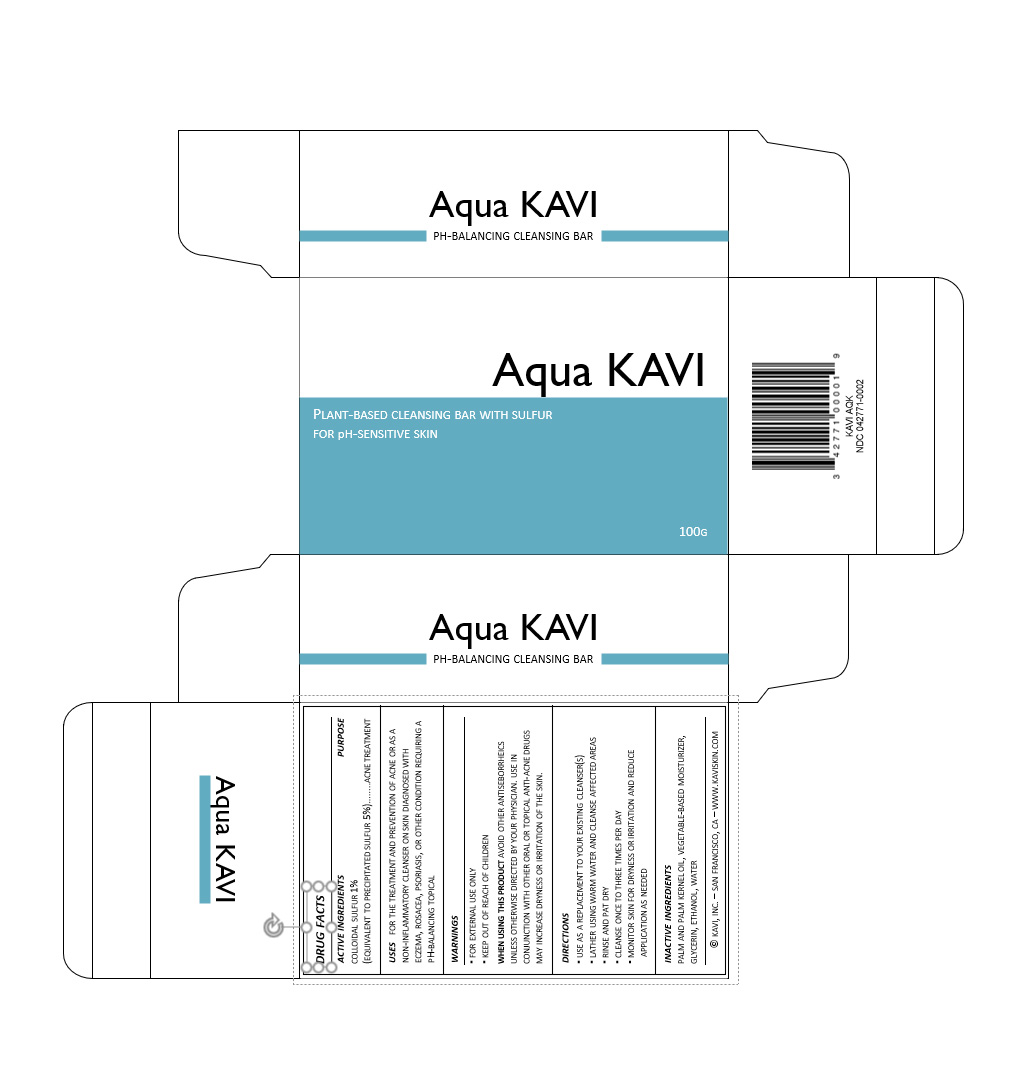
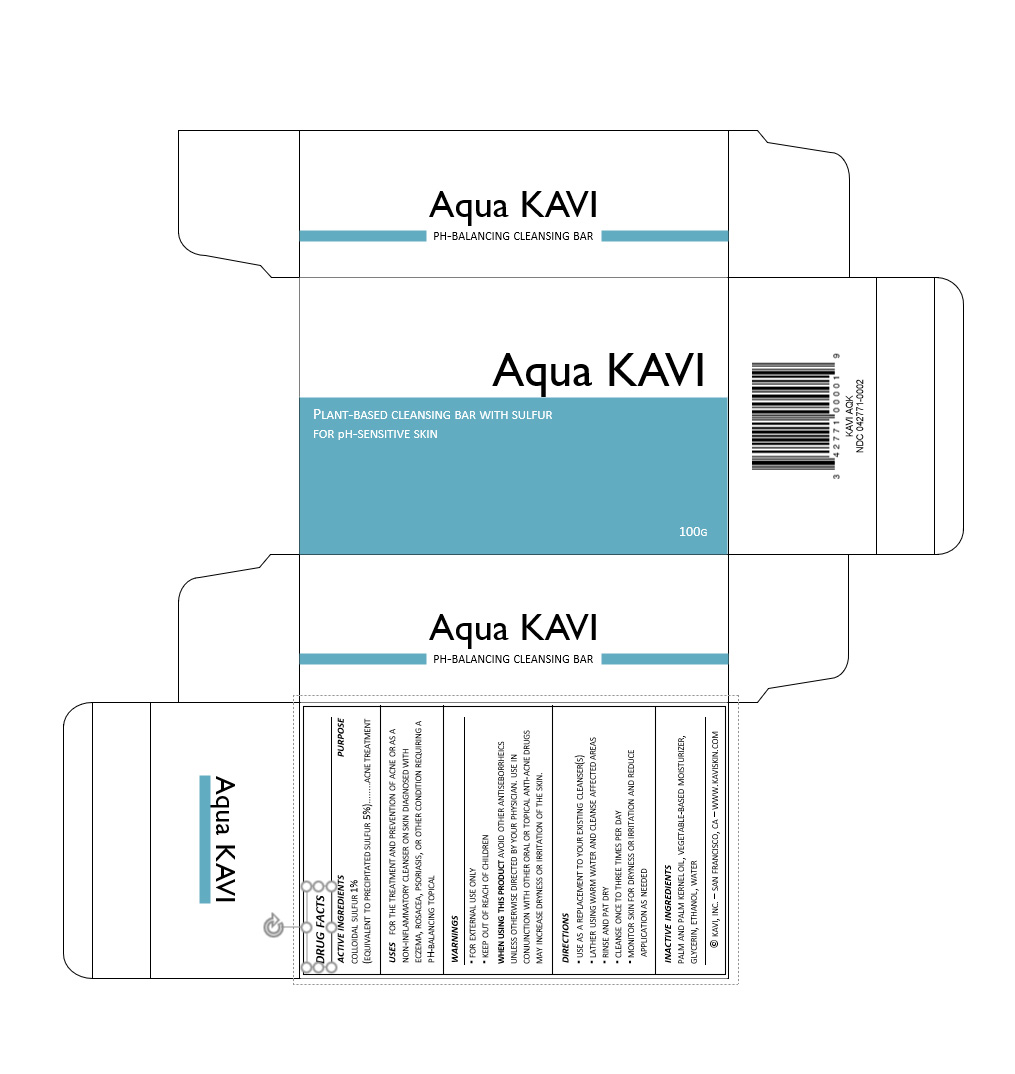
Label: AQUA KAVI- sulfur soap
-
Contains inactivated NDC Code(s)
NDC Code(s): 42771-0002-1 - Packager: KAVI Skin Solutions, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 20, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- active ingredients, purpose
-
Indications & Usage
uses
for the treatment and prevention of oily, acne-prone skindirections
▪ use as a replacement to your existing cleanser(s)
▪ lather using warm water and cleanse affected areas
▪ rinse and pat dry
▪ cleanse once to three times per day
▪ monitor skin for dryness or irritation and reduce application as needed - Dosage and Administration
- Warnings
- inactive ingredients
- Purpose
- Keep out of reach of children
-
Active Ingredients, Uses, Warnings, Directions, Inactive Ingredients
drug facts
active ingredients/purpose
colloidal sulfur 1%
(equivalent to precipitated sulfur 5%)…...acne treatment
usesfor the treatment and prevention of acne or as a non-inflammatory cleanser on skin diagnosed with eczema, rosacea, psoriasis, or other condition requiring a pH-balancing topical
warnings
▪ for external use only
▪ keep out of reach of childrenwhen using this product avoid other antiseborrheics unless otherwise directed by your physician. use in conjunction with other oral or topical anti-acne drugs may increase dryness or irritation of the skin.
directions
▪ use as a replacement to your existing cleanser(s)
▪ lather using warm water and cleanse affected areas
▪ rinse and pat dry
▪ cleanse once to three times per day
▪ monitor skin for dryness or irritation and reduce application as neededinactive ingredients
palm and palm kernel oil, vegetable-based moisturizer, glycerin, ethanol, water
-
INGREDIENTS AND APPEARANCE
AQUA KAVI
sulfur soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42771-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 1 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED PALM OIL (UNII: 257THB963H) 90 g in 100 g SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) 1 g in 100 g ALCOHOL (UNII: 3K9958V90M) 1 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) 1 g in 100 g 1,2-BUTANEDIOL (UNII: RUN0H01QEU) 0.5 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) 0.25 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42771-0002-1 1 g in 1 BOX; Type 0: Not a Combination Product 05/01/2003 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 05/01/2003 Labeler - KAVI Skin Solutions, Inc. (179144683)