Label: AVON HEXASHIELD TRIPLE ANTIBIOTIC WITH PAIN RELIEF- bacitracin zinc, neomycin sulfate, polymyxin b sulfate, pramoxine hcl ointment
- NDC Code(s): 43136-100-01
- Packager: Tai Guk Pharm. Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 3, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients (in each gram)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
-
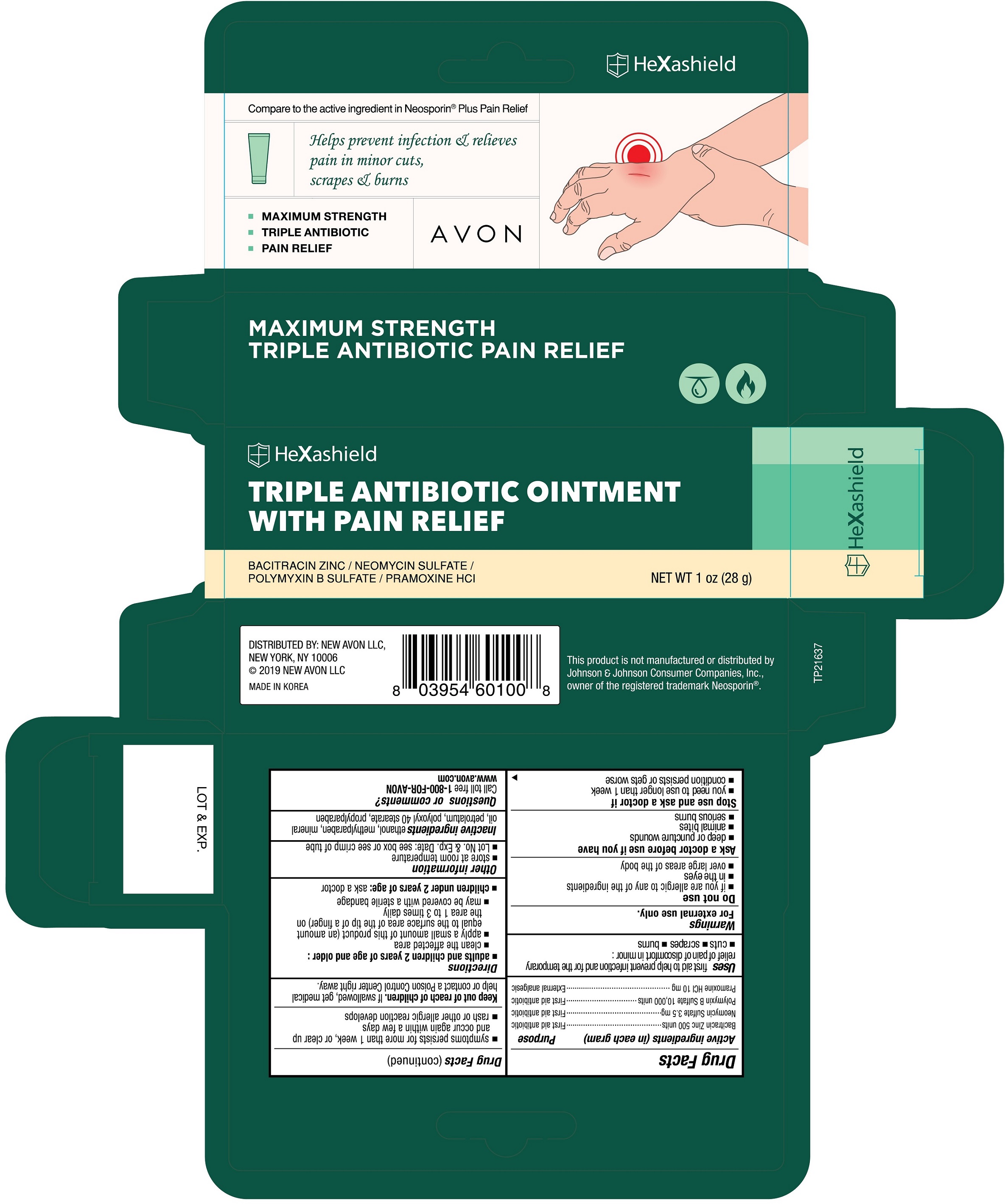
Principal Display Panel
Compare to the active ingredient in Neosporin ® Plus Pain Relief
Helps prevent infection & relieves pain in minor cuts, scrapes & burns
- MAXIMUM STRENGTH
- TRIPLE ANTIBIOTIC
- PAIN RELIEF
AVON
HeXashield
TRIPLE ANTIBIOTIC OINTMENT WITH PAIN RELIEF
BACITRACIN ZINC / NEOMYCIN SULFATE / POLYMYXIN B SULFATE / PRAMOXINE HCl
NET WT 1 oz (28g)

-
INGREDIENTS AND APPEARANCE
AVON HEXASHIELD TRIPLE ANTIBIOTIC WITH PAIN RELIEF
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, pramoxine hcl ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43136-100 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43136-100-01 1 in 1 CARTON 07/31/2019 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 07/31/2019 Labeler - Tai Guk Pharm. Co., Ltd. (689060246) Registrant - New Avon LLC (080143520) Establishment Name Address ID/FEI Business Operations Tai Guk Pharma. Co., Ltd. 689060246 manufacture(43136-100)


