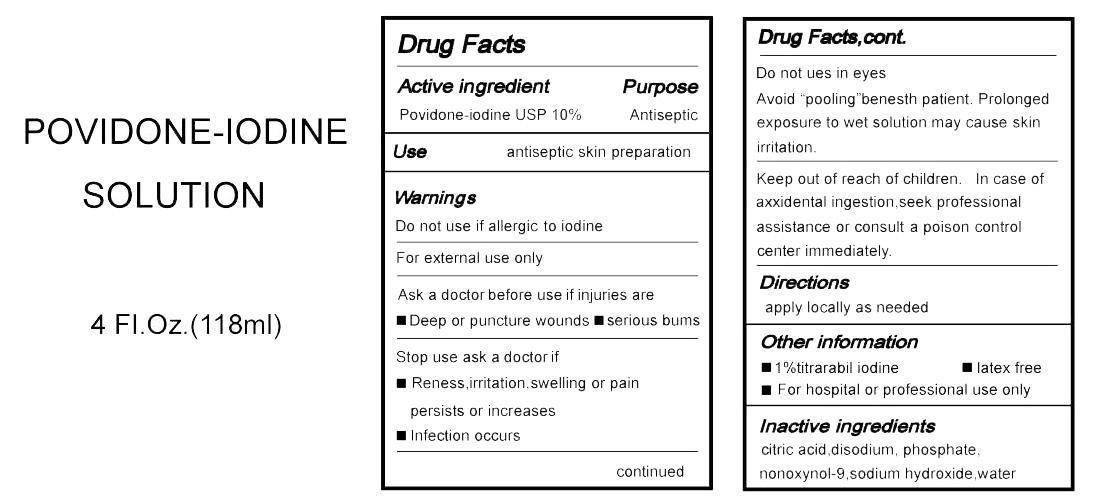
Label: POVIDONE-IODINE SOLUTION- povidone-iodine solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 58488-006-01 - Packager: Tongzhou Ruihong Medical Products Factory
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 26, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
-
Warnings
Do not use if allergic to iodine.
For External use only.
Ask a doctor before use if injuries are
--Deep or puncture wounds
--serious bums
stop use ask a doctor if
--reness ,irritation ,swelling or pain persists or increases
--infection occurs
Do not use in eyes
Avoid "pooling "benesth patient.Prolonged exposure to wet solution may cause skin irrtation.
- Directions
- Other information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
POVIDONE-IODINE SOLUTION
povidone-iodine solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58488-006 Route of Administration PERCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 0.01 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM HYDROXIDE (UNII: 55X04QC32I) NONOXYNOL-9 (UNII: 48Q180SH9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58488-006-01 1201 g in 1 PACKAGE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 05/20/2014 Labeler - Tongzhou Ruihong Medical Products Factory (421312726) Establishment Name Address ID/FEI Business Operations Tongzhou Ruihong Medical Products Factory 421312726 manufacture(58488-006)