Label: NOVOLIN 70/30- human insulin injection, suspension
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-3474-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 0169-1837
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 11, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PATIENT PACKAGE INSERT
-
PURPOSE
Important:
Know your insulin. Do not change the type of insulin you use unless told to do so by your healthcare provider. The amount of insulin you take as well as the best time for you to take your insulin may need to change if you take a different type of insulin.
Make sure that you know the type and strength of insulin that is prescribed for you.
Read the Patient Information leaflet that comes with Novolin 70/30 before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your diabetes or your treatment. Make sure you know how to manage your diabetes. Ask your healthcare provider if you have any questions about managing your diabetes.
What is Novolin 70/30?
Novolin 70/30 is a man-made insulin (recombinant DNA origin) which is a mixture of 70% NPH, Human Insulin Isophane Suspension and 30% Regular, Human Insulin Injection that is structurally identical to the insulin produced by the human pancreas that is used to control high blood sugar in patients with diabetes mellitus.
- DO NOT USE
-
ASK YOUR DOCTOR
Tell your healthcare provider:
- about all of your medical conditions. Medical conditions can affect your insulin needs and your dose of Novolin 70/30.
- if you are pregnant or breastfeeding. You and your healthcare provider should talk about the best way to manage your diabetes while you are pregnant or breastfeeding. Novolin 70/30 has not been studied in pregnant or nursing women.
- about all of the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. Many medicines can affect your blood sugar levels and your insulin needs. Your Novolin 70/30 dose may need to change if you take other medicines.
Know the medicines you take. Keep a list of your medicines with you to show all your healthcare providers when you get a new medicine.
-
DOSAGE AND ADMINISTRATION
How should I take Novolin 70/30?
Only use Novolin 70/30 if it appears cloudy or milky. There may be air bubbles. This is normal. If the precipitate (the white deposit at the bottom of the vial) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the vial, do not use it, and call Novo Nordisk at 1-800-727-6500. This insulin should not be used if the liquid in the vial remains clear after the vial has been gently rotated.
Novolin 70/30 comes in:
- 10 mL vials (small bottles) for use with syringe
-
INDICATIONS AND USAGE
Read the instructions for use that come with your Novolin 70/30 product. Talk to your healthcare provider if you have any questions. Your healthcare provider should show you how to inject Novolin 70/30 before you start taking it. Follow your healthcare provider’s instructions to make changes to your insulin dose.
- Take Novolin 70/30 exactly as prescribed.
- Novolin 70/30 is an intermediate-acting insulin. The effects of Novolin 70/30 start working ½ hour after injection.
- The greatest blood sugar lowering effect is between 2 and 12 hours after the injection. This blood sugar lowering may last up to 24 hours.
- While using Novolin 70/30, any change of insulin should be made cautiously and only under medical supervision. Doses of oral anti-diabetic medicines may also need to change, if your insulin is changed.
- Do not mix Novolin 70/30 with any insulins.
- Inject Novolin 70/30 into the skin of your stomach area, upper arms, buttocks or upper legs. Novolin 70/30 may affect your blood sugar levels sooner if you inject it into the skin of your stomach area. Never inject Novolin 70/30 into a vein or into a muscle.
- Change (rotate) your injection site within the chosen area (for example, stomach or upper arm) with each dose. Do not inject into the same spot for each injection.
- If you take too much Novolin 70/30, your blood sugar may fall low (hypoglycemia). You can treat mild low blood sugar (hypoglycemia) by drinking or eating something sugary right away (fruit juice, sugar candies, or glucose tablets). It is important to treat low blood sugar (hypoglycemia) right away because it could get worse and you could pass out (become unconscious). If you pass out, you will need help from another person or emergency medical services right away, and will need treatment with a glucagon injection or treatment at a hospital. See “What are the possible side effects of Novolin 70/30?” for more information on low blood sugar (hypoglycemia).
- If you forget to take your dose of Novolin 70/30, your blood sugar may go too high (hyperglycemia). If high blood sugar (hyperglycemia) is not treated it can lead to diabetic ketoacidosis, which can lead to serious problems, like loss of consciousness (passing out), coma or even death. Follow your healthcare provider’s instructions for treating high blood sugar (hyperglycemia), and talk to your healthcare provider if high blood sugar is a problem for you. Severe or continuing high blood sugar (hyperglycemia) requires prompt evaluation and treatment by your healthcare provider. Know your symptoms of high blood sugar (hyperglycemia) and diabetic ketoacidosis which may include:
-
- increased thirst
- frequent urination and dehydration
- confusion or drowsiness
- loss of appetite
- fruity smell on breath
- high amounts of sugar and ketones in your urine
- nausea, vomiting (throwing up) or stomach pain
- a hard time breathing
- Check your blood sugar levels. Ask your healthcare provider how often you should check your blood sugar levels for hypoglycemia (too low blood sugar) and hyperglycemia (too high blood sugar).
Your insulin dosage may need to change because of:
- illness
- stress
- other medicines you take
- change in diet
- change in physical activity or exercise
- surgery
See the end of this patient information for instructions about preparing and giving the injection.
What should I avoid while using Novolin 70/30?
- Alcohol. Alcohol, including beer and wine, may affect your blood sugar when you take Novolin 70/30.
-
Driving and operating machinery. You may have difficulty concentrating or reacting if you have low blood sugar (hypoglycemia). Be careful when you drive a car or operate machinery. Ask your healthcare provider if it is alright to drive if you often have:
- low blood sugar
- decreased or no warning signs of low blood sugar
-
ADVERSE EFFECTS
What are the possible side effects of Novolin 70/30?
Low blood sugar (hypoglycemia). Symptoms of hypoglycemia (low blood sugar) may include:
- sweating
- dizziness or lightheadedness
- shakiness
- hunger
- fast heart beat
- tingling of lips and tongue
- trouble concentrating or confusion
- blurred vision
- slurred speech
- anxiety, irritability or mood changes
- headache
Severe low blood sugar (hypoglycemia) can cause unconsciousness (passing out), seizures, and death. Know your symptoms of low blood sugar. Follow your healthcare provider’s instructions for treating low blood sugar. Talk to your healthcare provider if low blood sugar is a problem for you.
- Serious allergic reaction (whole body reaction). Get medical help right away if you develop a rash over your whole body, have trouble breathing, a fast heartbeat, or sweating.
- Reactions at the injection site (local allergic reaction). You may get redness, swelling, and itching at the injection site. If you keep having skin reactions, or they are serious, talk to your healthcare provider. You may need to stop using Novolin 70/30 and use a different insulin. Do not inject insulin into skin that is red, swollen, or itchy.
- Skin thickens or pits at the injection site (lipodystrophy). Change (rotate) where you inject your insulin to help prevent these skin changes from happening. Do not inject insulin into this type of skin.
- Swelling of your hands and feet
- Vision changes
- Low potassium in your blood (hypokalemia)
These are not all of the possible side effects from Novolin 70/30. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
-
STORAGE AND HANDLING
How should I store Novolin 70/30?
All Unopened Novolin 70/30:
- Keep all unopened Novolin 70/30 in the refrigerator between 36° to 46°F (2° to 8°C).
- Do not freeze. Do not use Novolin 70/30 if it has been frozen.
- If refrigeration is not possible, the unopened vial may be kept at room temperature for up to 6 weeks (42 days), as long as it is kept at or below 77°F (25°C).
- Keep unopened Novolin 70/30 in the carton to protect from light.
Novolin 70/30 in use:
Vials
- Keep at room temperature below 77°F (25°C) for up to 6 weeks (42 days).
- Keep vials away from direct heat or light.
- Throw away an opened vial after 6 weeks (42 days) of use, even if there is insulin left in the vial.
- Unopened vials can be used until the expiration date on the Novolin 70/30 label, if the medicine has been stored in a refrigerator.
General advice about Novolin 70/30
Novolin 70/30 is used for the treatment of diabetes only. Medicines are sometimes prescribed for conditions that are not mentioned in the patient leaflet. Do not use Novolin 70/30 for a condition for which it was not prescribed. Do not give Novolin 70/30 to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Novolin 70/30. If you would like more information about Novolin 70/30 or diabetes, talk with your healthcare provider. For more information, call 1-800-727-6500 or visit www.novonordisk-us.com.
Helpful information for people with diabetes is published by the American Diabetes Association, 1701 N Beauregard Street, Alexandria, VA 22311 and on www.diabetes.org.
- ACTIVE INGREDIENTS
-
INACTIVE INGREDIENTS
Novolin 70/30 ingredients include:
- Zinc chloride
- Sodium hydroxide
- Phenol
- Disodium phosphate dihydrate
- Metacresol
- Glycerol
- Hydrochloric acid
- Protamine sulfate
- Water for injections
All Novolin 70/30 vials are latex-free.
Date of issue: May 14, 2010
Version: 5
Novolin® and Novo Nordisk® are trademarks of Novo Nordisk A/S.
© 2005-2010 Novo Nordisk A/S
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about Novolin 70/30 contact:
Novo Nordisk Inc.
100 College Road West
Princeton, New Jersey 08540
Patient Instructions for Use
Novolin® 70/30 10 mL vial (100 Units/mL, U-100)
Before starting, gather all of the supplies that you will need to use for preparing and giving your insulin injection.
Never re-use syringes and needles.
How should I use the Novolin 70/30 vial?
- Check to make sure that you have the correct type of insulin.
- Look at the vial and the insulin. The insulin should be a cloudy or milky suspension. The tamper-resistant cap should be in place before the first use. If the cap had been removed before your first use of the vial, or if the precipitate (the white deposit at the bottom of the vial) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the vial, do not use it, and call Novo Nordisk at 1-800-727-6500.
- Wash your hands with soap and water. If you clean your injection site with an alcohol swab, let the injection site dry before you inject. Talk with your healthcare provider about how to rotate injection sites and how to give an injection.
- If you are using a new vial, pull off the tamper-resistant cap. Wipe the rubber stopper with an alcohol swab.
- Roll the vial gently 10 times in your hands to mix it. This procedure should be carried out with the vial in a horizontal position. The rolling procedure must be repeated until the suspension appears uniformly white and cloudy. Shaking right before the dose is drawn into the syringe may cause bubbles or froth, which could cause you to draw up the wrong dose of insulin.
- Pull back the plunger on the syringe until the black tip reaches the marking for the number of units you will inject.
- Push the needle through the rubber stopper of the vial, and push the plunger all the way in to force air into the vial.
- Turn the vial and syringe upside down and slowly pull the plunger back to a few units beyond the correct dose.
- If there are any air bubbles, tap the syringe gently with your finger to raise the air bubbles to the top. Then slowly push the plunger to the marking for your correct dose. This process should move any air bubbles present in the syringe back into the vial.
- Check to make sure you have the right dose of Novolin 70/30 in the syringe.
- Pull the syringe with needle out of the vial’s rubber stopper.
- Your doctor should tell you if you need to pinch the skin before inserting the needle. This can vary from patient to patient so it is important to ask your doctor if you did not receive instructions on pinching the skin. Insert the needle into the skin. Press the plunger of the syringe to inject the insulin. When you are finished injecting the insulin, pull the needle out of your skin. You may see a drop of Novolin 70/30 at the needle tip. This is normal and has no effect on the dose you just received. If you see blood after you take the needle out of your skin, press the injection site lightly with a piece of gauze or an alcohol wipe. Do not rub the area.
- After your injection, do not recap the needle. Place used syringes, needles and used insulin vials in a disposable puncture-resistant sharps container, or some type of hard plastic or metal container with a screw on cap such as a detergent bottle or coffee can.
- Ask your healthcare provider about the right way to throw away used syringes and needles. There may be state or local laws about the right way to throw away used syringes and needles. Do not throw away used needles and syringes in household trash or recycle.
Information for the patient
Novolin®70/30 PenFill®
70% NPH, Human Insulin Isophane Suspension and
30% Regular, Human Insulin Injection
(recombinant DNA origin)
3 mL Disposable Cartridge
(300 units per cartridge)
100 units/mL (U-100)
Please read this leaflet carefully before using this product.
Please note the special directions under PREPARING THE INJECTION.
Novolin® 70/30 PenFill® 3 mL is designed for use with Novo Nordisk® 3 mL PenFill cartridge compatible insulin delivery devices, with or without the addition of a NovoPen® 3 PenMate®, and NovoFine® disposable needles.
PenFill® cartridge is for single-person use only. See IMPORTANT NOTES section.
-
WARNING
WARNING
ANY CHANGE OF INSULIN SHOULD BE MADE CAUTIOUSLY AND ONLY UNDER MEDICAL SUPERVISION. CHANGES IN PURITY, STRENGTH, BRAND (MANUFACTURER), TYPE (REGULAR, NPH, LENTE®, ETC.), SPECIES (BEEF, PORK, BEEF-PORK, HUMAN), AND/OR METHOD OF MANUFACTURE (RECOMBINANT DNA VERSUS ANIMAL-SOURCE INSULIN) MAY RESULT IN THE NEED FOR A CHANGE IN DOSAGE.
SPECIAL CARE SHOULD BE TAKEN WHEN THE TRANSFER IS FROM A STANDARD BEEF OR MIXED SPECIES INSULIN TO A PURIFIED PORK OR HUMAN INSULIN. IF A DOSAGE ADJUSTMENT IS NEEDED, IT WILL USUALLY BECOME APPARENT EITHER IN THE FIRST FEW DAYS OR OVER A PERIOD OF SEVERAL WEEKS. ANY CHANGE IN TREATMENT SHOULD BE CAREFULLY MONITORED.
PLEASE READ THE SECTIONS “INSULIN REACTION AND SHOCK” AND “DIABETIC KETOACIDOSIS AND COMA” FOR SYMPTOMS OF HYPOGLYCEMIA (LOW BLOOD GLUCOSE) AND HYPERGLYCEMIA (HIGH BLOOD GLUCOSE).
INSULIN USE IN DIABETES
Your physician has explained that you have diabetes and that your treatment involves injections of insulin or insulin therapy combined with an oral antidiabetic medicine. Insulin is normally produced by the pancreas, a gland that lies behind the stomach. Without insulin, glucose (a simple sugar made from digested food) is trapped in the bloodstream and cannot enter the cells of the body. Some patients who don’t make enough of their own insulin, or who cannot use the insulin they do make properly, must take insulin by injection in order to control their blood glucose levels.
Each case of diabetes is different and requires direct and continued medical supervision. Your physician has told you the type, strength and amount of insulin you should use and the time(s) at which you should inject it, and has also discussed with you a diet and exercise schedule. You should contact your physician if you experience any difficulties or if you have questions.
TYPES OF INSULINS
Standard and purified animal insulins as well as human insulins are available. Standard and purified insulins differ in their degree of purification and content of noninsulin material. Standard and purified insulins also vary in species source; they may be of beef, pork, or mixed beef and pork origin. Human insulin is identical in structure to the insulin produced by the human pancreas, and thus differs from animal insulins. Insulins vary in time of action; see PRODUCT DESCRIPTION for additional information. Your physician has prescribed the insulin that is right for you; be sure you have purchased the correct insulin and check it carefully before you use it.
PRODUCT DESCRIPTION
This package contains five (5) Novolin® 70/30 PenFill® 3 mL cartridges. Novolin 70/30 is a mixture of 70% NPH, Human Insulin Isophane Suspension and 30% Regular, Human Insulin Injection (recombinant DNA origin). The concentration of this product is 100 units of insulin per milliliter. It is a cloudy or milky suspension of human insulin with protamine and zinc. The insulin substance (the cloudy material) settles at the bottom of the cartridge, therefore, the cartridge must be rotated up and down as described under PREPARING THE INJECTION so that the contents are uniformly mixed before a dose is given. Novolin 70/30 has an intermediate duration of action. The effect of Novolin 70/30 begins approximately ½ hour after injection. The effect is maximal between 2 and approximately 12 hours. The full duration of action may last up to 24 hours after injection.
The time course of action of any insulin may vary considerably in different individuals, or at different times in the same individual. Because of this variation, the time periods listed here should be considered as general guidance only.
This human insulin (recombinant DNA origin) is structurally identical to the insulin produced by the human pancreas. This human insulin is produced by recombinant DNA technology utilizing Saccharomyces cerevisiae (bakers’ yeast) as the production organism.
INSULIN DELIVERY SYSTEMS
These Novolin 70/30 PenFill 3 mL cartridges are designed for use with Novo Nordisk® 3 mL PenFill cartridge compatible insulin delivery devices, with or without the addition of a NovoPen® 3 PenMate®, and NovoFine® disposable needles.
STORAGE
Insulin should be stored in a cold (36° - 46°F [2° - 8°C]) place, preferably in a refrigerator, but not in the freezer. Do not let it freeze. Keep Novolin 70/30 PenFill cartridges in the carton so that they will stay clean and protected from light. The Novolin 70/30 PenFill cartridge that you are currently using should not be refrigerated but should be kept as cool as possible (below 86°F [30°C]) and away from direct heat and light. Unrefrigerated Novolin 70/30 PenFill cartridges must be discarded 10 days after the first use, even if they still contain Novolin 70/30 insulin.
Never use PenFill cartridges after the expiration date which is printed on the label and carton.
Never use any Novolin 70/30 PenFill cartridge if the precipitate (the white deposit) has become lumpy or granular in appearance or has formed a deposit of solid particles on the wall of the cartridge. This insulin should not be used if the liquid in the cartridge remains clear after it has been mixed.
IMPORTANT
Failure to follow the antiseptic measures listed below may lead to infections at the injection site.
– Disposable needles are for single use; they should be used only once and destroyed.
– Clean your hands and the injection site with soap and water or with alcohol.
– Wipe the rubber stopper on the insulin cartridge with an alcohol swab.
PREPARING THE INJECTION
Never place a single-use disposable needle on your insulin delivery device until you are ready to give an injection, and remove it immediately after the injection. If the needle is not removed, some liquid may be expelled from the cartridge causing a change in the insulin concentration (strength).
The cloudy material in an insulin suspension will settle to the bottom of the cartridge, so the contents must be mixed before injection. These Novolin PenFill cartridges contain a glass ball to aid mixing.
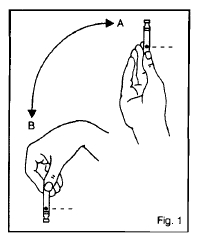
Figure 1
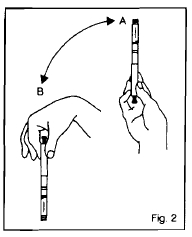
Figure 2
When using a new cartridge, turn the cartridge up and down between positions A and B – See Figure 1. Do this at least 10 times until the liquid appears uniformly white and cloudy. Assemble your insulin delivery device following the directions in your instruction manual. For subsequent injections when a cartridge is already in the device, turn the device up and down between positions A and B - See Figure 2. Do this at least 10 times until the liquid appears uniformly white and cloudy. Follow the directions in your insulin delivery device instruction manual.
Note: Never initiate a new injection unless there is sufficient insulin in the cartridge to ensure proper mixing (the glass ball needs adequate room for movement to mix the suspension).
PenFill cartridges may contain a small amount of air bubbles. To prevent an injection of air and to make certain that a full dose of insulin is injected, an air shot must be done before each injection. Directions for performing an air shot are provided in your insulin delivery device instruction manual.
GIVING THE INJECTION
- The following areas are suitable for subcutaneous insulin injection: thighs, upper arms, buttocks, abdomen. Do not change areas without consulting your physician. The actual point of injection should be changed each time; injection sites should be about an inch apart.
- The injection site should be clean and dry. Pinch up skin area to be injected and hold it firmly.
- Hold the device like a pencil and push the needle quickly and firmly into the pinched-up area.
- Release the skin and push the push button all the way in to inject insulin beneath the skin. After the injection, the needle should remain under the skin for at least 6 seconds. Keep the push button fully depressed until the needle is withdrawn from the skin. This will ensure that the full dose has been injected.
- Do not inject into a muscle unless your physician has advised it. You should never inject insulin into a vein. Follow the directions for use of your insulin delivery device.
- Remove the needle. If slight bleeding occurs, press lightly with a dry cotton swab for a few seconds - do not rub.
Note: Use the injection technique recommended by your physician.
USAGE IN PREGNANCY
It is particularly important to maintain good control of your diabetes during pregnancy and special attention must be paid to your diet, exercise and insulin regimens. If you are pregnant or nursing a baby, consult your physician or nurse educator.
INSULIN REACTION AND SHOCK
Insulin reaction (hypoglycemia) occurs when the blood glucose falls very low. This can happen if you take too much insulin, miss or delay a meal, exercise more than usual, or work too hard without eating, or become ill (especially with vomiting or fever). Hypoglycemia can also happen if you combine insulin therapy and other medications that lower blood glucose, such as oral antidiabetic agents or other prescription and over-the-counter drugs. The first symptoms of an insulin reaction usually come on suddenly. They may include a cold sweat, fatigue, nervousness or shakiness, rapid heartbeat, or nausea. Personality change or confusion may also occur. If you drink or eat something right away (a glass of milk or orange juice, or several sugar candies), you can often stop the progression of symptoms. If symptoms persist, call your physician - an insulin reaction can lead to unconsciousness. If a reaction results in loss of consciousness, emergency medical care should be obtained immediately. If you have had repeated reactions or if an insulin reaction has led to a loss of consciousness, contact your physician. Severe hypoglycemia can result in temporary or permanent impairment of brain function and death.
In certain cases, the nature and intensity of the warning symptoms of hypoglycemia may change. A few patients have reported that after being transferred to human insulin, the early warning symptoms of hypoglycemia were less pronounced than they had been with animal-source insulin.
DIABETIC KETOACIDOSIS AND COMA
Diabetic ketoacidosis may develop if your body has too little insulin. The most common causes are acute illness or infection or failure to take enough insulin by injection. If you are ill, you should check your urine for ketones. The symptoms of diabetic ketoacidosis usually come on gradually, over a period of hours or days, and include a drowsy feeling, flushed face, thirst and loss of appetite. Notify your physician right away if the urine test is positive for ketones (acetone) or if you have any of these symptoms. Fast, heavy breathing and rapid pulse are more severe symptoms and you should have medical attention right away. Severe, sustained hyperglycemia may result in diabetic coma and death.
ADVERSE REACTIONS
A few people with diabetes develop red, swollen and itchy skin where the insulin has been injected. This is called a “local reaction” and it may occur if the injection is not properly made, if the skin is sensitive to the cleansing solution, or if you are allergic to the insulin being used. If you have a local reaction, tell your physician.
Generalized insulin allergy occurs rarely, but when it does it may cause a serious reaction, including skin rash over the body, shortness of breath, fast pulse, sweating, and a drop in blood pressure. If any of these symptoms develop, you should seek emergency medical care.
If severe allergic reactions to insulin have occurred (i.e., generalized rash, swelling or breathing difficulties) you should be skin-tested with each new insulin preparation before it is used.
IMPORTANT NOTES
- A change in the type, strength, species or purity of insulin could require a dosage adjustment. Any change in insulin should be made under medical supervision.
- To avoid possible transmission of disease, PenFill cartridge should not be shared.
- Before use, check that the PenFill cartridge is intact (e.g. no cracks). Do not use if any damage is visible, or if the part of the rubber piston that you see is wider than the white bar code band.
- You may have learned how to test your urine or your blood for glucose. It is important to do these tests regularly and to record the results for review with your physician or nurse educator.
- If you have an acute illness, especially with vomiting or fever, continue taking your insulin. If possible, stay on your regular diet. If you have trouble eating, drink fruit juices, regular soft drinks, or clear soups; if you can, eat small amounts of bland foods. Test your urine for glucose and ketones and, if possible, test your blood glucose. Note the results and contact your physician for possible insulin dose adjustment. If you have severe and prolonged vomiting, seek emergency medical care.
- You should always carry identification which states that you have diabetes.
- Always ask your physician or pharmacist before taking any drug.
- Do not try to refill a PenFill cartridge.
Always consult your physician if you have any questions about your condition or the use of insulin.
Helpful information for people with diabetes is published by the American Diabetes Association, 1660 Duke Street, Alexandria, VA 22314
Date of issue: November 18, 2005
Protected by U.S. Patent No. 6,126,646 and No. 5,693,027 and Des. 347,894 and other U.S. Patents Pending, recommended for use with Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery devices, with or without a NovoPen 3 PenMate, and Novo Nordisk pen needles.
© 2004/2005 Novo Nordisk Inc.
Novo Nordisk® , Novolin®, PenFill®, NovoPen®, PenMate®, NovoFine® and Lente® are registered trademarks owned by Novo Nordisk A/S.
Novo Nordisk Inc.
Princeton, NJ 08540
Call 1-800-727-6500 for additional information
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Patient Instructions for Use
Novolin 70/30® InnoLet®
Novolin® 70/30 InnoLet® directions for use
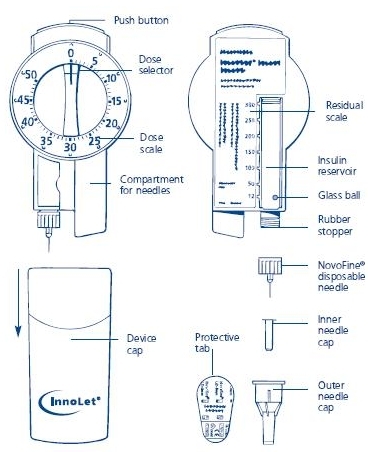
Novolin® 70/30 InnoLet® is a disposable dial-a-dose insulin delivery system able to deliver 1-50 units in increments of 1 unit. Novolin® 70/30 InnoLet® is designed and recommended for use with NovoFine® single-use needles. Novolin® 70/30 InnoLet® is not recommended for the blind or severely visually impaired patients without the assistance of a sighted individual trained in the proper use of this product.
Please read these instructions completely before using this device.
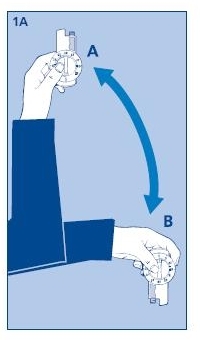
1. Preparing the Novolin® 70/30 InnoLet®:
Pull off the device cap.
1A. Turn the Novolin® 70/30 InnoLet® up and down between positions A and B so the glass ball is moved from one end of the insulin reservoir to the other. Do this at least 10 times, until the liquid appears uniformly white and cloudy.
To ensure even mixing of the remaining insulin there must be at least 12 units of insulin left in the reservoir. If there are less than 12 units left, do not use the Novolin® 70/30 InnoLet®.
Wipe rubber stopper with an alcohol swab.
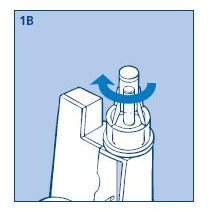
1B. Remove the protective tab from the disposable needle and screw the needle onto the Novolin® 70/30 InnoLet®. Never place a disposable needle on your Novolin® 70/30 InnoLet® until you are ready to give an injection. Remove the needle immediately after use. If the needle is not removed, some liquid may be expelled from the Novolin® 70/30 InnoLet® causing a change in insulin concentration (strength).
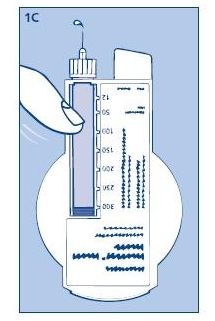
1C. Giving the air shot prior to each injection:
Small amounts of air may collect in the needle and insulin reservoir during normal use. To avoid the injection of air and ensure proper dosing, dial 2 units by turning the dose selector clockwise. Hold the Novolin® 70/30 InnoLet® with the needle up and tap the Novolin® 70/30 InnoLet® gently with your finger so any air bubbles collect in the top of the reservoir. Remove both the plastic outer and inner needle caps.
With the needle pointing up, press the push button as far as it will go and the dose selector returns to zero. See if a drop of insulin appears at the needle tip (see fig. 1C). If not, repeat the procedure until insulin appears.
Before the first use of Novolin® 70/30 InnoLet® you may need to perform up to 6 air shots to get a drop of insulin at the needle tip. If you need to make more than 6 air shots, do not use, and return the product to Novo Nordisk. A small air bubble may remain but it will not be injected because the operating mechanism prevents the reservoir from being completely emptied.
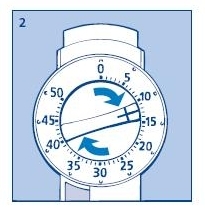
2. Setting the dose
Always check that the push button is fully depressed and the dose selector is set to zero. Hold the Novolin® 70/30 InnoLet® in front of you and dial the dose selector clockwise to set the required dose. Do not put your hand over the push button when dialing the dose. If the button is not allowed to rise freely, insulin will be pushed out of the needle. When setting your dose, you will hear a click for every single unit dialed. Do not rely on this clicking sound as a means of determining your dose. If you have set a wrong dose, simply dial the dose selector forward or backwards until the right number of units has been set.
50 units is the maximum dose.
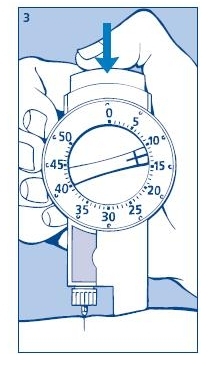
3. Giving the injection
Use the injection technique recommended by your doctor. Check that you have set the proper dose and depress the push button as far as it will go. Make sure not to block the dose selector while injecting as the dose selector must be allowed to return to zero when you press the push button. When depressing the push button you may hear a clicking sound. Do not rely on this clicking sound as a means of confirming delivery of your dose.
After making the injection, unscrew the needle and discard appropriately. Replace the device cap.
Health care professionals, relatives, and other care-givers should follow general precautionary measures for removal and disposal of needles to eliminate the risk of unintended needle penetration.
For additional information see Giving the injection on the reverse side of this insert.
Subsequent injections
Always check that the push button is fully depressed before using the Novolin® 70/30 InnoLet® again. If not, turn the dose selector until the push button is completely down. Then proceed as stated under steps 1-3. The numbers on the insulin reservoir can be used to estimate the amount of insulin left in the Novolin® 70/30 InnoLet®. These numbers are not used for measuring the insulin dose.
You cannot set a dose greater than the number of units remaining in the reservoir.
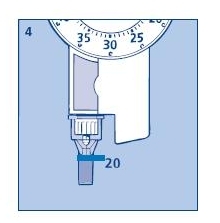
4. Function check
If you think that your Novolin® 70/30 InnoLet® is not working properly, follow this procedure:
a. Screw on a new NovoFine needle.
b. Perform air shot as described in section 1C.
c. Put the outer needle cap onto the needle.
d. Dispense 20 units into the needle cap.
The insulin will fill the lower part of the cap (as shown in fig. 4).
If the Novolin® 70/30 InnoLet® has released too much or too little insulin, repeat the test. If it happens again, contact Novo Nordisk and do not use your Novolin® 70/30 InnoLet®.
5. Important notes
a. If you need to perform more than 6 air shots before the first use of Novolin® 70/30 InnoLet® to get a drop of insulin at the needle tip, do not use.
b. Remember to perform an air shot before each injection (see fig. 1C).
c. Care should be taken not to drop the Novolin® 70/30 InnoLet® or subject it to impact.
d. Remember to keep the Novolin® 70/30 InnoLet® that you are currently using with you; don’t leave it in a car or other location where extremes of temperature can occur.
e. Novolin® 70/30 InnoLet® is designed and recommended for use with NovoFine disposable needles.
f. Never place a disposable needle on the Novolin® 70/30 InnoLet® until you are ready to use it. Remove the needle immediately after use.
g. Discard the used Novolin® 70/30 InnoLet® carefully, without the needle attached.
h. Always carry a spare Novolin® 70/30 InnoLet® with you in case your Novolin® 70/30 InnoLet® is damaged or lost.
i. Novo Nordisk cannot be held responsible for adverse reactions occurring as a consequence of using the insulin delivery system with products that are not recommended by Novo Nordisk.
-
KEEP OUT OF REACH OF CHILDREN
j. Keep Novolin® 70/30 InnoLet® out of the reach of children.
© 2002/2008 Novo Nordisk A/S
Call 800-727-6500 for additional information.
Novo Nordisk Inc.
Princeton, NJ 08540
www.novonordisk-us.com
Manufactured by
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
Novo Nordisk®, Novolin®, Lente®, NovoFine® and InnoLet® are trademarks owned by Novo Nordisk A/S
U.S. Patents Nos. 5,947,934, 6,074,372, 6,110,149, 6,302,869, 6,524,280, 6,379,339, 6,582,404, and other U.S. patents pending.
-
Principal Display Panel - 10 mL Vial
Novolin® 70/30 10 mL Vial
70% NPH, Human Insulin Isophane Suspension and
30% Regular, Human Insulin Injection
(recombinant DNA origin)
NDC 54868-3474-0

10 mL
Use with U-100 insulin syringes only
Shake carefully before use
Keep in cold place
Avoid freezing
Additional barcode labeling by:
Physicians Total Care, Inc.
Tulsa, Oklahoma 74146
-
INGREDIENTS AND APPEARANCE
NOVOLIN 70/30
human insulin injection, suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54868-3474(NDC:0169-1837) Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INSULIN HUMAN (UNII: 1Y17CTI5SR) (INSULIN HUMAN - UNII:1Y17CTI5SR) INSULIN HUMAN 100 [USP'U] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) METACRESOL (UNII: GGO4Y809LO) PHENOL (UNII: 339NCG44TV) PROTAMINE SULFATE (UNII: 0DE9724IHC) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) ZINC CHLORIDE (UNII: 86Q357L16B) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-3474-0 1 in 1 CARTON 1 10 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019991 01/11/1995 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 relabel

