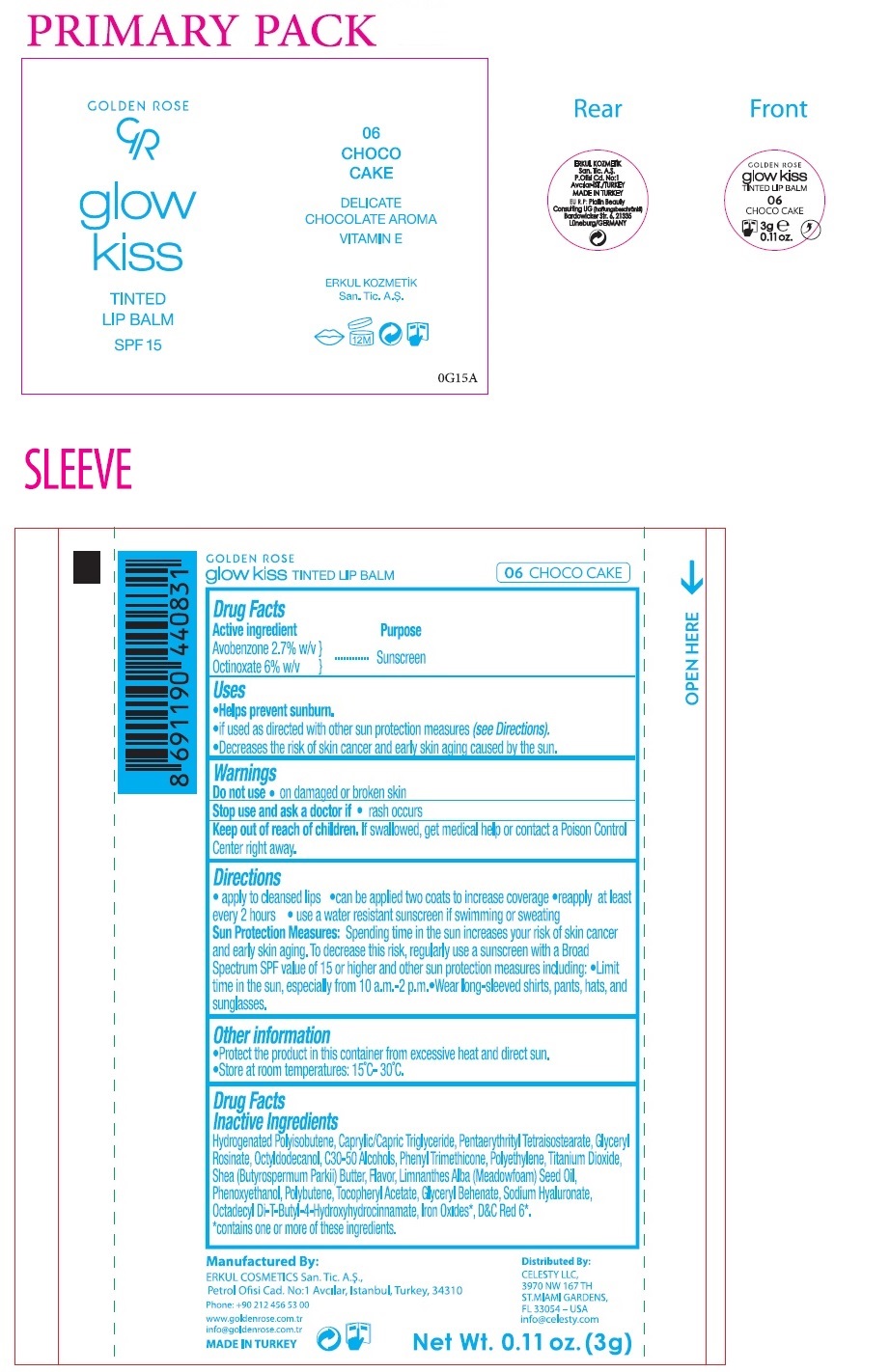
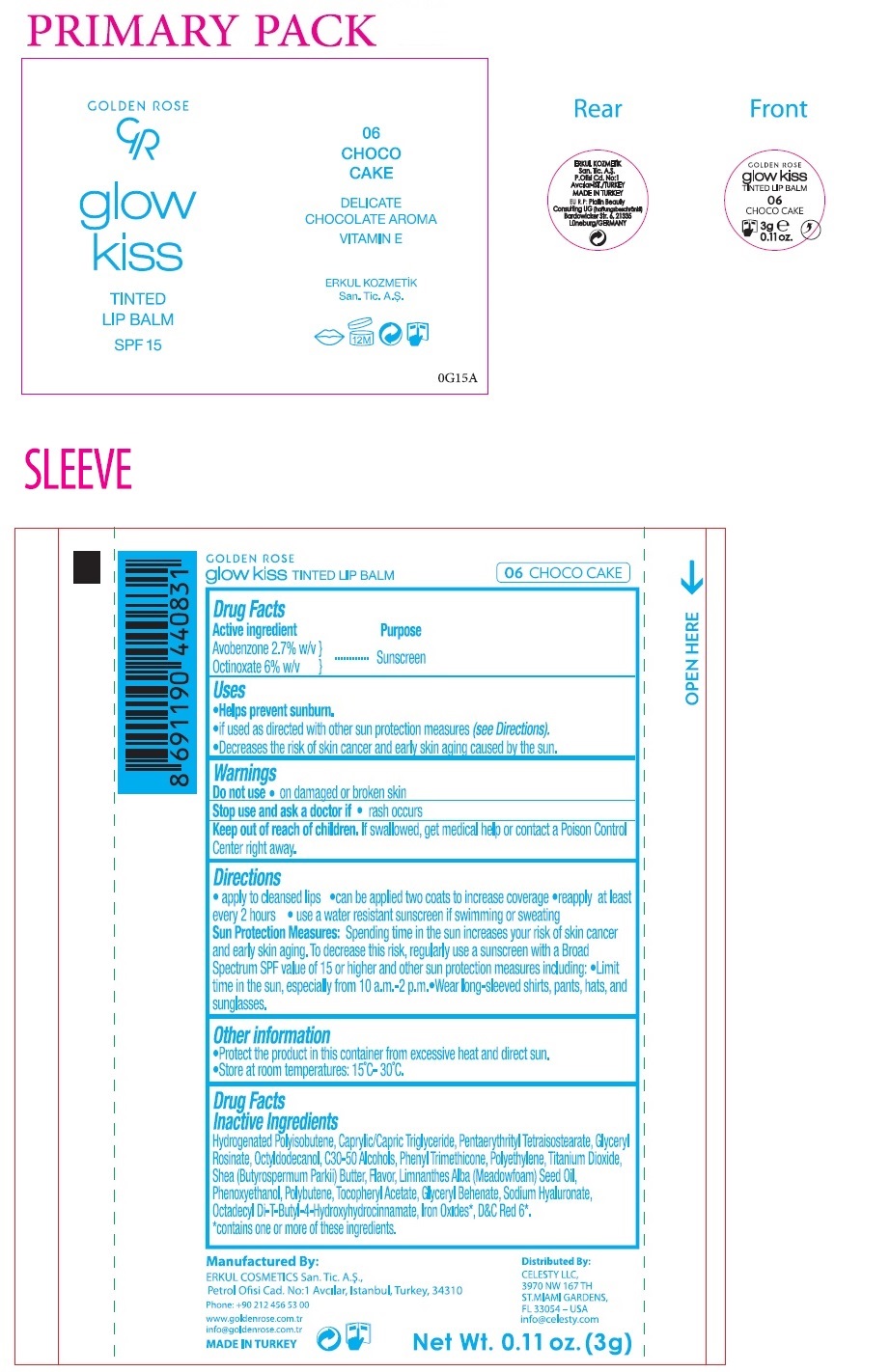
Label: GOLDEN ROSE GLOW KISS TINTED LIP BALM CHOCO CAKE- avobenzone, octinoxate lipstick
- NDC Code(s): 82715-108-01
- Packager: ERKUL KOZMETIK SANAYI VE TICARET ANONIM SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
• apply to cleansed lips • can be applied two coats to increase coverage • reapply at least every 2 hours • use a water resistant sunscreen if swimming or sweating
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: • Limit time in the sun, especially from 10 a.m. - 2 p.m • Wear long-sleeved shirts, pants, hats, and sunglasses. - Other information
-
Drug FactsInactive ingredients
Hydrogenated Polyisobutene, Caprylic/Capric Triglyceride, Pentaerythrityl Tetraisostearate, Glyceryl Rosinate, Octyldodecanol, C30-50 Alcohols, Phenyl Trimethicone, Polyethylene, Titanium Dioxide, Shea (Butyrospermum parkii) Butter, Flavor, Limnanthes Alba (Meadowfoam) Seed Oil, Phenoxyethanol, Polybutene, Tocopheryl Acetate, Glyceryl Behenate, Sodium Hyaluronate, Octadecyl Di-T-Butyl -4-Hydroxyhydrocinnamate, Iron Oxides*, D&C Red 6*.
* contains one or more of these ingredients. -
SPL UNCLASSIFIED SECTION
SPF 15
DELICATE
CHOCOLATE AROMA
VITAMIN EManufactured By:
ERKUL COSMETICS San. Tic. A.S.,
Petrol Ofisi Cad. No:1 Avcılar, Istanbul, Turkey, 34310Phone: +90 212 456 53 00
www.goldenrose.com.tr
info@goldenrose.com.trMADE IN TURKEY
Distributed By:
CELESTY LLC,
3970 NW 167 TH
ST.MIAMI GARDENS,
FL 33054 – USA
info@celesty.comEU R.P: Platin Beauty Consulting UG
(haftungsbeschränkt)
Bardowicker Str. 6, 21335
Lüneburg/GERMANY - Packaging
-
INGREDIENTS AND APPEARANCE
GOLDEN ROSE GLOW KISS TINTED LIP BALM CHOCO CAKE
avobenzone, octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82715-108 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.7 g in 100 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PENTAERYTHRITYL TETRAISOSTEARATE (UNII: 9D7IK5483F) GLYCERYL ROSINATE (UNII: SD112V492J) OCTYLDODECANOL (UNII: 461N1O614Y) C30-50 ALCOHOLS (UNII: 4MCU204619) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHEA BUTTER (UNII: K49155WL9Y) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERYL MONOBEHENATE (UNII: A626UU0W2A) HYALURONATE SODIUM (UNII: YSE9PPT4TH) OCTADECYL DI-TERT-BUTYL-4-HYDROXYHYDROCINNAMATE (UNII: V88J661G2P) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) D&C RED NO. 6 (UNII: 481744AI4O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82715-108-01 3 g in 1 TUBE; Type 0: Not a Combination Product 07/22/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/22/2022 Labeler - ERKUL KOZMETIK SANAYI VE TICARET ANONIM SIRKETI (525225637) Establishment Name Address ID/FEI Business Operations ERKUL KOZMETIK SANAYI VE TICARET ANONIM SIRKETI 525225637 manufacture(82715-108)