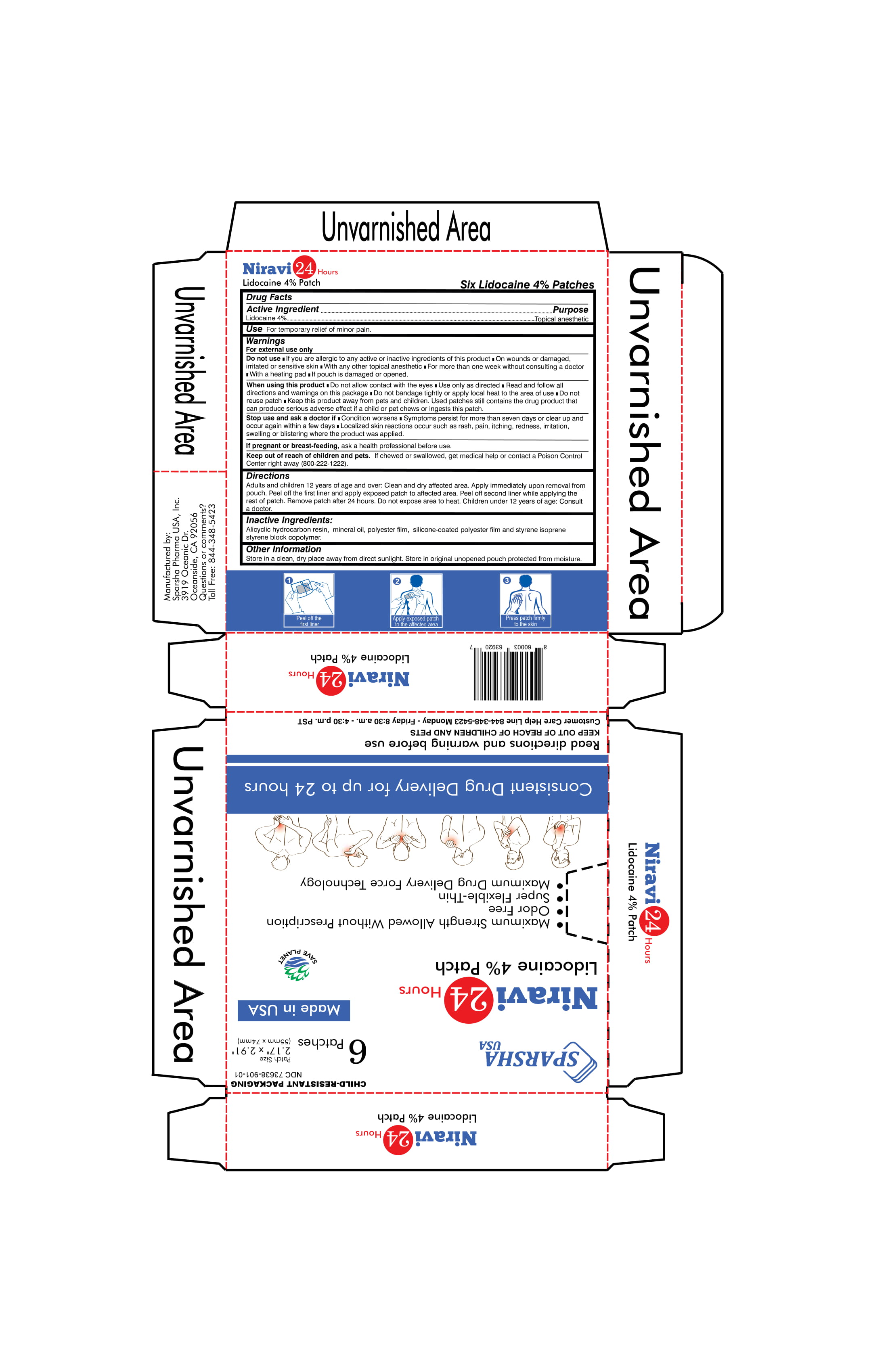
Label: NIRAVI 24 HOURS LIDOCAINE 4 % PATCH- lidocaine patch
- NDC Code(s): 73638-901-01
- Packager: Sparsha Pharma USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
-
When using this product
• Do not allow contact with the eyes
• Use only as directed
• Read and follow all directions and warnings on this package
• Do not bandage tightly or apply local heat to the area of use
• Do not reuse patch
• Keep this product away from pets and children. Used patches still contains the drug product that can produce serious adverse effect if child or pet chews or ingest this patch. - Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Directions
Adults and children 12 years of age and over: Clean and dry affected area. Apply immediately upon removal from pouch. Peel off the first liner and apply exposed patch to affected area. Peel off second liner while applying the rest of patch. Remove patch after 24 hours. Do not expose area to heat.
Children under 12 years of age: Consult a doctor.
- Inactive Ingredients
- Other information
- Questions ?
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
NIRAVI 24 HOURS LIDOCAINE 4 % PATCH
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73638-901 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (STYRENE/ISOPRENE 15/85) (UNII: 1SSZ6HXE7P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73638-901-01 6 in 1 CARTON 06/30/2021 1 1 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/30/2021 Labeler - Sparsha Pharma USA, Inc. (079365856) Registrant - Sparsha Pharma USA, Inc. (079365856) Establishment Name Address ID/FEI Business Operations Sparsha Pharma USA, Inc. 079365856 manufacture(73638-901)