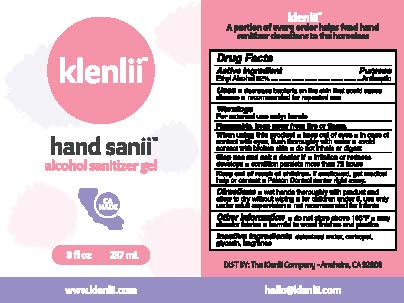
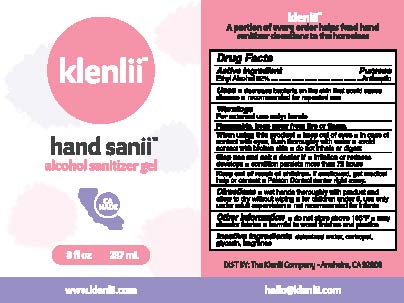
Label: KLENLII HAND SANII ALCOHOL SANITIZER- ethyl alcohol gel
-
NDC Code(s):
78016-102-11,
78016-102-12,
78016-102-14,
78016-102-16, view more78016-102-18
- Packager: Klenlii Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 9, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
-
WARNINGS
FOR EXTERNAL USE ONLY: HANDS
FLAMMABLE, KEEP AWAY FROM FIRE OR FLAME.
WHEN USING THIS PRODUCT
- KEEP OUT OF EYES
- IN CASE OF CONTACT WITH EYES, FLUSH THOROUGHLY WITH WATER
- AVOID CONTACT WITH BROKEN SKIN
- DO NOT INHALE OR DIGEST
STOP USE AND ASK DOCTOR IF
- IRRITATION OR REDNESS DEVELOPS
- CONDITION PERSISTS MORE THAN 72 HOURS
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- INACTIVE INGREDIENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
KLENLII HAND SANII ALCOHOL SANITIZER
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:78016-102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 63 mL in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:78016-102-18 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2020 2 NDC:78016-102-14 118 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 05/27/2020 3 NDC:78016-102-16 476 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2020 4 NDC:78016-102-11 3785 mL in 1 JUG; Type 0: Not a Combination Product 05/27/2020 5 NDC:78016-102-12 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/27/2020 Labeler - Klenlii Company (117278596)