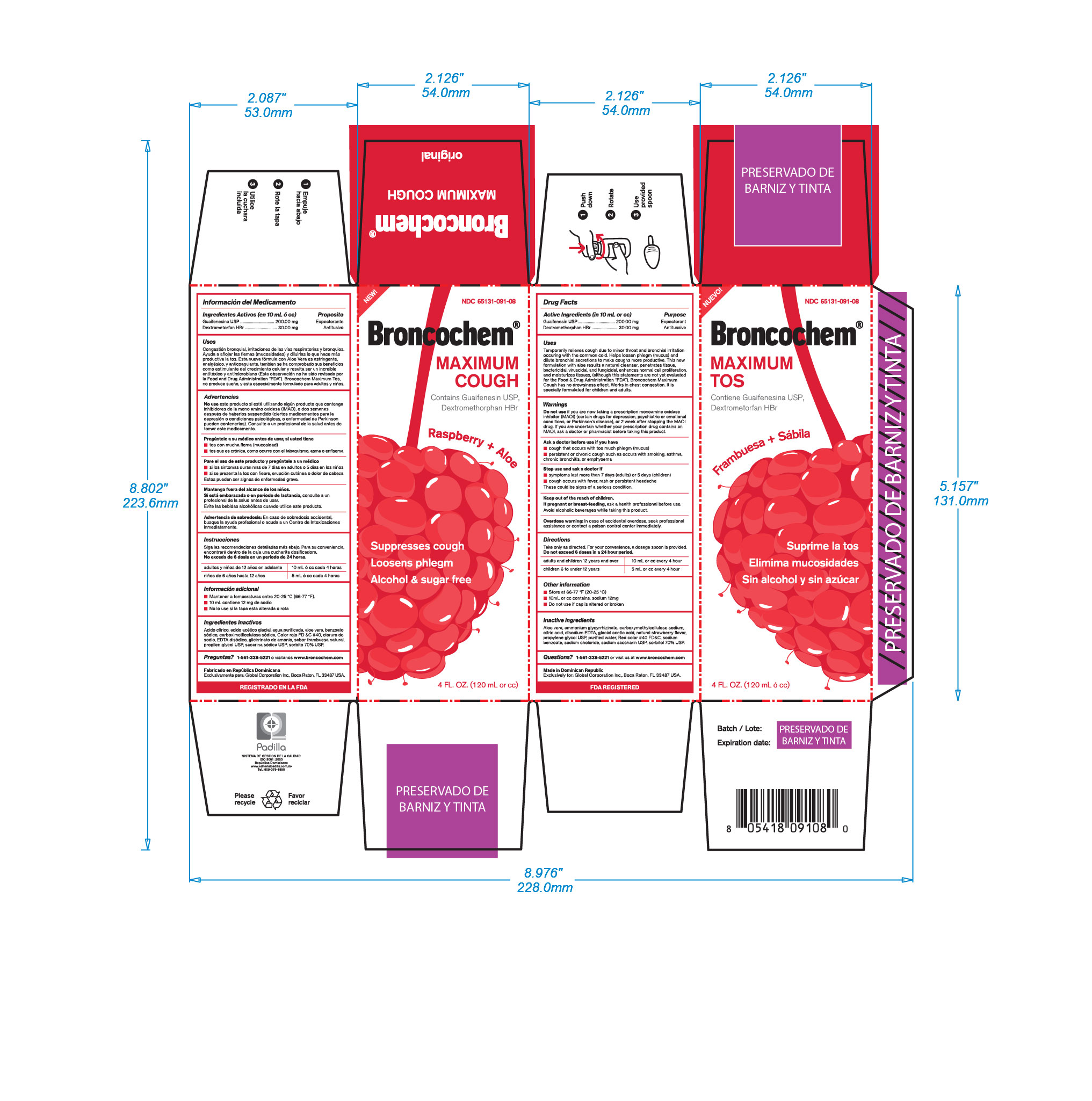
Label: BRONCOCHEM MAXIMUM COUGH- dextromethorphan hbr-gyaifenesin syrup
- NDC Code(s): 65131-091-08
- Packager: LABORATORIO MAGNACHEM INTERNATIONAL SRL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 19, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Warning Secction
Do not take this product for persitent or chronic cough such as occurs with smoking, asthma, or emphysema or if cough is accompainied with excessive phlegm (mucus) unless directed by a doctor. Persistent cough may be the sign of a serious condition. Stop use and dask a doctor if symptoms persist or last more than 5 days (children) or 7 days (adults), tends to recur, is accompained or followed by fever, headache, rash, swelling, nausea or vomiting, consult a doctor. Do not take this product if you are hypersensitive to any of the ingredients. As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product. Avoid alcoholic beverages while taking this product.
- Active Ingredients
- Purpose
- Keep out of the reach of children
-
Indications and Usage
Temporarily relieves cough due to minor throat and bronchial irritation occuring with common cold.
Helps loosen phlegm (mucus) and dilute bronchial secretions to make coughs more productive.
This new formulation with aloe results a natural cleanser, penetrates tissue, bactericidal, viruscidal, and fungicidal, enhances normal cell proliferation and moisturizes tissues (this statements are not yet evaluated by the Food and Drugs Administration "FDA")
Broncochem Maximum Cough has not drowsiness effect.
Work in chest congestion, its specially formulated for children and adults.
- Dossage and Administration
-
Drug Interactions Section
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI), (certain drugs for depression, psychiatric or emotional conditions or Parkinsons disease), or 2 weeks after stopping the MAOI drug. Id you are uncertain ehether your prescription drug contains an MAOI, consult a health professional before taking this product
- Inactive Ingredient Section
- Package Label Principal Display Panel
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRONCOCHEM MAXIMUM COUGH
dextromethorphan hbr-gyaifenesin syrupProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65131-091 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 10 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 4 mg in 10 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 20 mg in 10 mL CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 6.8 mg in 10 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 12 mg in 10 mL FD&C RED NO. 40 (UNII: WZB9127XOA) 0.508 mg in 10 mL DISODIUM HEDTA (UNII: KME849MC7A) 5 mg in 10 mL AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) 20 mg in 10 mL SACCHARIN SODIUM (UNII: SB8ZUX40TY) 30 mg in 10 mL ACETIC ACID (UNII: Q40Q9N063P) 0.016 mL in 10 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 1.6 mL in 10 mL RASPBERRY (UNII: 4N14V5R27W) 0.04 mL in 10 mL SORBITOL (UNII: 506T60A25R) 2 mL in 10 mL ALOE VERA LEAF (UNII: ZY81Z83H0X) 20 mg in 10 mL WATER (UNII: 059QF0KO0R) 10 mL in 10 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65131-091-08 1 in 1 BOX 12/30/2016 1 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 12/30/2016 Labeler - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) Registrant - LABORATORIO MAGNACHEM INTERNATIONAL SRL (871446100) Establishment Name Address ID/FEI Business Operations LABORATORIO MAGNACHEM INTERNATIONAL SRL 871446100 manufacture(65131-091)