Label: COLISTAT- docusate sodium tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 69975-750-05 - Packager: Amvilab LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 16, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
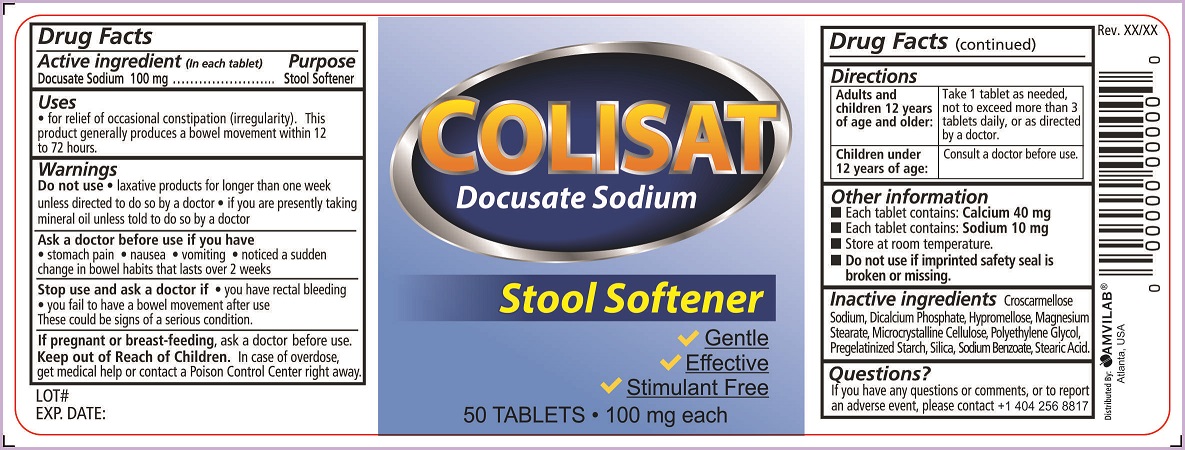
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- laxative products for longer than one week unless directed to do so by a doctor
- if you are presently taking mineral oil unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- Directions
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
COLISTAT
docusate sodium tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69975-750 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BENZOATE (UNII: OJ245FE5EU) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white Score 2 pieces Shape ROUND (Round Biconvex with bisect) Size 11mm Flavor Imprint Code GPI;S1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69975-750-05 50 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 07/16/2015 Labeler - Amvilab LLC (006092439) Registrant - Gemini Pharmaceuticals, Inc. dba Plus Pharma (055942270) Establishment Name Address ID/FEI Business Operations Gemini Pharmaceuticals, Inc. dba Plus Pharma 055942270 manufacture(69975-750)