Label: D TIME SINUS- acetaminophen and phenylephrine hydrochloride capsule, liquid filled
-
Contains inactivated NDC Code(s)
NDC Code(s): 68210-1470-5, 68210-1470-6 - Packager: SPIRIT PHARMACEUTICALS,LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 26, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 6 doses in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). Ask a doctor or pharmacist before using with other drugs if you are not sure
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- to make a child sleep
Ask a doctor before use if you have
- liver disease
- heart disease
- thyroid disease
- diabetes
- high blood pressure
- trouble urinating due to enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
When using these products
- do not use more than directed
In addition, when using NyQuil Sinus:
- excitability may occur, especially in children
- marked drowsiness may occur
- avoid alcoholic drinks
- be careful when driving a motor vehicle or operating machinery
- alcohol, sedatives, and tranquilizers may increase drowsiness
-
Directions
- take only as recommended - see Overdose warning
- do not exceed 6 doses per 24 hours
NyQuil Sinus OR DayQuil Sinus
adults and children
12 years and over2 LiquiCaps with
water every 4 hourschildren 2 to under 12 years ask a doctor children under 2 years do not use - when using other DayQuil or NyQuil products, carefully read each label to insure correct dosing
- Other information
-
Inactive ingredients
NyQuil Sinus FD&C Blue No. 1, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol special, titanium dioxide.
DayQuil Sinus FD&C Yellow No. 6, gelatin, glycerin, polyethylene glycol, povidone, propylene glycol, purified water, sorbitol special, titanium dioxide.
-
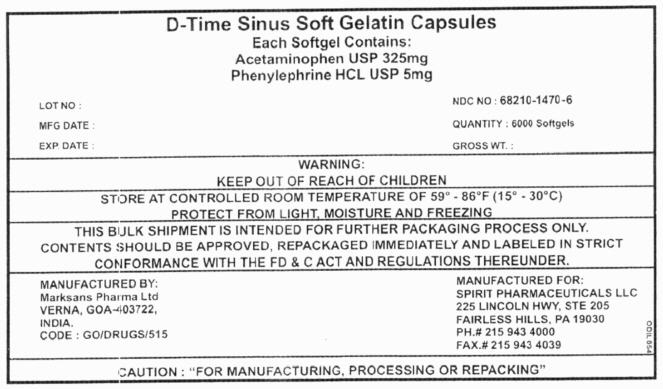
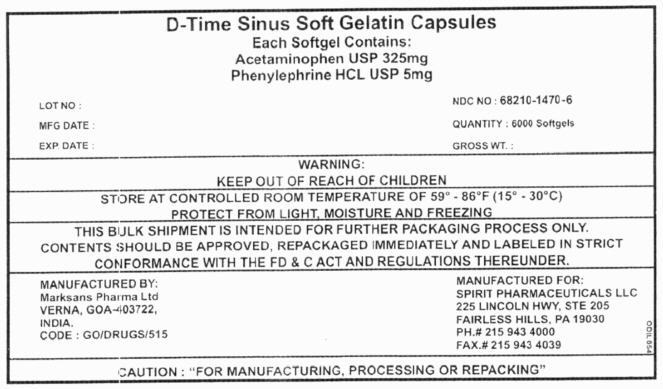
PRINCIPAL DISPLAY PANEL - 6000 Softgels
D-Time Sinus Soft Gelatin Capsules
Each Softgel Contains:
Acetaminophen USP 325mg
Phenylephrine HCL USP 5mgLOT NO : NDC NO : 68210-1470-6 MFG DATE : QUANTITY : 6000 Softgels EXP. DATE : GROSS WT. : WARNING:
KEEP OUT OF REACH OF CHILDRENSTORE AT CONTROLLED ROOM TEMPERATURE OF 59° - 86°F (15° - 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS BULK SHIPMENT IS INTENDED FOR FURTHER PACKAGING PROCESS ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE FD & C ACT AND REGULATIONS THEREUNDER.MANUFACTURED BY:
Marksans Pharma Ltd
VERNA, GOA-403722,
INDIA.
CODE : GO/DRUGS/515MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS, PA 19030
PH.# 215 943 4000
FAX.# 215 943 4039CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
-
INGREDIENTS AND APPEARANCE
D TIME SINUS
acetaminophen and phenylephrine hydrochloride capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-1470 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GELATIN (UNII: 2G86QN327L) POVIDONE (UNII: FZ989GH94E) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE Score no score Shape OVAL Size 18mm Flavor Imprint Code 129 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-1470-5 1 in 1 DRUM 1 5000 in 1 BAG 2 NDC:68210-1470-6 1 in 1 DRUM 2 6000 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 03/01/2010 Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011)