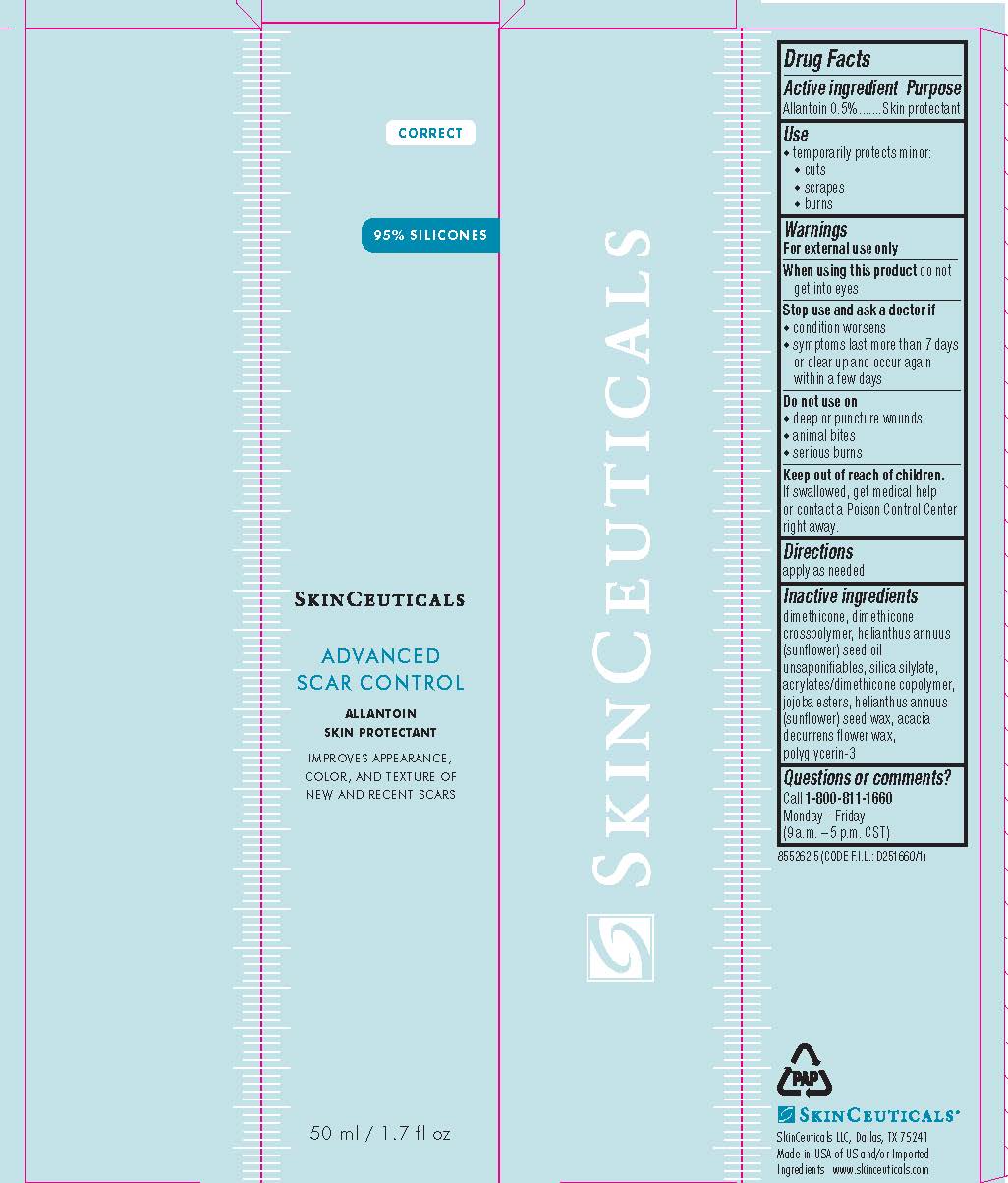
Label: SKINCEUTICALS ADVANCED SCAR CONTROL SKIN PROTECTANT- allantoin gel
- NDC Code(s): 49967-783-01, 49967-783-02, 49967-783-03
- Packager: L'Oreal USA Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Do not use on
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
- Directions
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKINCEUTICALS ADVANCED SCAR CONTROL SKIN PROTECTANT
allantoin gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49967-783 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) SUNFLOWER OIL (UNII: 3W1JG795YI) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49967-783-01 1 in 1 CARTON 06/01/2021 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:49967-783-02 4 mL in 1 TUBE; Type 0: Not a Combination Product 06/01/2021 3 NDC:49967-783-03 1 in 1 CARTON 06/01/2021 3 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/01/2021 Labeler - L'Oreal USA Products Inc (002136794) Establishment Name Address ID/FEI Business Operations Beauty Manufacturing Solutions Corp. 783200723 manufacture(49967-783)