Label: READY CARE KIT- first aid kit kit
-
NDC Code(s):
59898-740-01,
59898-902-01,
65517-0001-1,
65517-0004-1, view more65517-0020-1
- Packager: Dukal Corporation
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: OTC monograph final
Drug Label Information
Updated September 23, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
Back Label
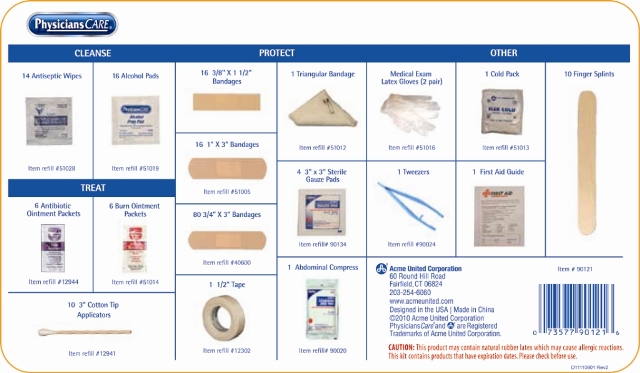
Physicians Care
CLEANSE
Antiseptics
14 Antiseptic Wipes
16 Alcohol PadsTREAT
6 Antibiotic Ointment Packets
PROTECT
6 Burn Ointment Packets
10 3" Cotton Tip Applicator16 3/8” x 1 1/2” Bandages
16 1” x 3” Bandages
80 3/4” x 3” Bandages
1 1/2" Tape
1 Triangular Bandage
4 3" x 3" Sterile Gauze Pads
1 Abdominal Compress
Other
Medical Exam Latex Gloves (2 Pair)
1 Tweezers
1 Cold Pack
1 First Aid Guide
10 Finger Splints
Acme United Corporation
60 Round Hill Road
Fairfield CT 06824
www.acmeunited.com
Designed in the USA | Made in China
CAUTION: This product may contain natural rubber latex which may cause allergic reactions.
This kit contains products that have expiration dates. Please check before use.
-
BZK Towelette Labeling
Reorder 855
NDC 65517-0004-1
Dukal
BZK TOWELETTE
Contains Benzalkonium Chloride
For External Use Only
Helps Prevent Infection
1 / Pouch
DUKAL CORPORATION
(631) 656-3800
Ronkonkoma, NY 11779 www.dukal.com
Made in China
Drug Facts
Active Ingredients..................... Benzalkonium Chloride 0.133% w/v
Purpose................................. First Aid Antiseptic
USE: Antiseptic Cleansing of face, hands and
body without soap and water. Airs dries in seconds
DO NOT USE: in the eyes or apply over large
areas of the body.
STOP USE: If irritation, redness or other symptoms
develop. Consult a doctor if the conditions persists
or gets worse.
CAUTION: Keep out of reach of children. If
swallowed get medical help or contact a Poison
Control Center right away.
DIRECTIONS: Tear open packet, unfold and use
as a washcloth.INACTIVE INGREDIENTS: Distilled Water
-
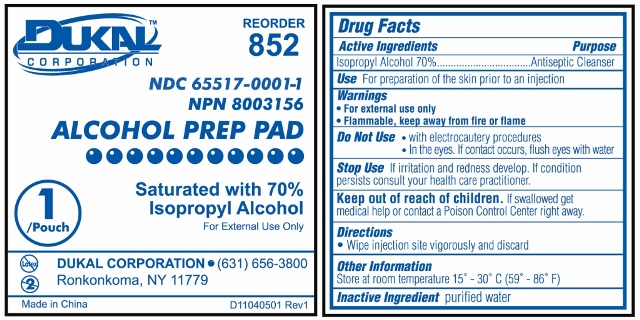
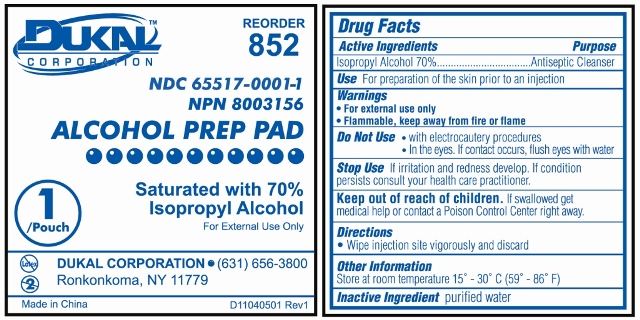
Prep Pad Labeling
Reorder 852
Dukal Corporation
NDC 65517-0001-1
NPN 80003156
ALCOHOL PREP PAD
Saturated with 70% Isopropyl Alcohol
For External Use Only
1 / Pouch
Dukal Corporation
Ronkonkoma, NY 11779
631-656-3800
www.dukal.com
Made in China
Drug Facts
Active Ingredients
Isopropyl Alcohol 70%
Purpose
Antiseptic Cleanser
Use
For Preparation of Skin prior to an injection
Warnings
- For External Use Only
- Flammable, Keep away from fire or flame
Do Not Use
- with electrocautery procedures
- In the Eyes. If contact occurs, flush eyes with water
Stop Use
If irritation and redness develop. If condition persists, consult your health care practitioner.Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.Directions
Wipe injection site vigorously and discard.Other Information
Store at Room Temperature 15 - 30 C (59 - 86 F)
Inactive Ingredient
purified water
-
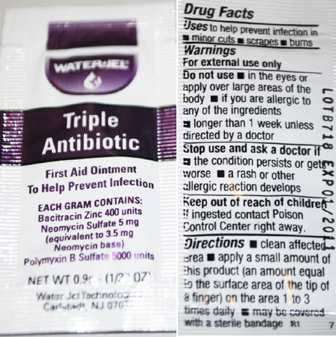
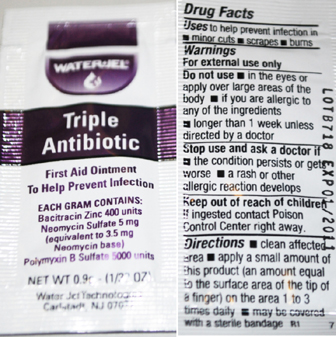
Triple Antibiotic Labeling
WaterJel
Triple Antibiotic
First Aid Ointment
To Help Prevent Infection
Each Gram contains
Bacitracin Zinc 400 units
Neomycin Sulphate 5 mg
(equivalent to 3.5 mg Neomycin base)
Polymyxin B Sulfate 5000 units
Water-Jel Technologies
Carlstadt, NJ 07072
Drug Facts
Uses to help prevent infection in
minor cuts, scrapes, burns
Warnings
For external use only
Do not use
in the eyes or apply over large areas of the body
If you are allergic to any of the ingredients
longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
the condition persists or gets worse
a rash or other allergic reaction develops
Keep out of reach of children
if ingested contact Poison Control Center right away
Directions
clean affected area apply a small amount of product
(an amount equal to the surface area of the tip of a finger)
on the area 1 to 3 times daily may be covered with a sterile bandage
-
First Aid Burn Cream Labeling
WaterJel
First Aid Burn Cream
Antiseptic Pain Relief with Aloe
Active Ingredients:
Benzalkonium Chloride 0.13%
Lidocaine HCL 0.5%
Water-Jel Technologies
Carlstadt, NJ 07072
Drug Facts
Purpose
First Aid Antiseptic, External analgesic
Uses
first aid to help prevent infection and for temporary
relief of pain an itching associated with minor cuts,
scrapes, burns
Warnings
For external use only
Do not use
in the eyes
in large quantities over raw or blistered areas or on
deep puncture wounds, animal bites, or serious burns
Keep out of reach of children
if ingested contact Poison Control Center right away
Directions
clean affected area apply a small amount not more
than 3 times daily may be covered with a sterile bandage
Other Information
Store at room temperature
- Ready Care Kit Front Label
- Ready Care Kit Back Label
- Prep Pad Label
- BZK Towelette Label
- Triple Antibiotic Label
- Burn Cream Label
-
INGREDIENTS AND APPEARANCE
READY CARE KIT
first aid kit kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NDC:65517-0020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0020-1 1 in 1 PACKAGE, COMBINATION Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 16 POUCH 6.4 mL Part 2 6 PACKET 5.4 g Part 3 6 PACKET 5.4 g Part 4 14 POUCH 19.6 mL Part 1 of 4 DUKAL ALCOHOL PREP PAD
isopropyl alcohol swabProduct Information Item Code (Source) NDC:65517-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.70 mL in 1.0 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0001-1 0.4 mL in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/2000 Part 2 of 4 WATER-JEL 3-IN1 ANTIBIOTIC
bacitracin zinc, polymyxin b sulfate and neomycin sulfate ointmentProduct Information Item Code (Source) NDC:59898-740 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN ZINC 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B SULFATE 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-740-01 0.9 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 01/01/2010 Part 3 of 4 FIRST AID BURN
lidocaine hydrochloride and benzalkonium chloride creamProduct Information Item Code (Source) NDC:59898-902 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) LIGHT MINERAL OIL (UNII: N6K5787QVP) STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) CETYL ALCOHOL (UNII: 936JST6JCN) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59898-902-01 0.9 g in 1 PACKET Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 01/01/2010 Part 4 of 4 BZK TOWELETTE
benzalkonium chloride swabProduct Information Item Code (Source) NDC:65517-0004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.00186 mL in 1.4 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 1.39814 mL in 1.4 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0004-1 1.4 mL in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 01/01/2006 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 05/01/2010 Labeler - Dukal Corporation (791014871)