Label: GOOD SENSE ALLERGY RELIEF- loratadine tablet
-
NDC Code(s):
0113-0612-39,
0113-0612-46,
0113-0612-49,
0113-0612-58, view more0113-0612-60, 0113-0612-65, 0113-0612-75, 0113-0612-88
- Packager: L. Perrigo Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 15, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
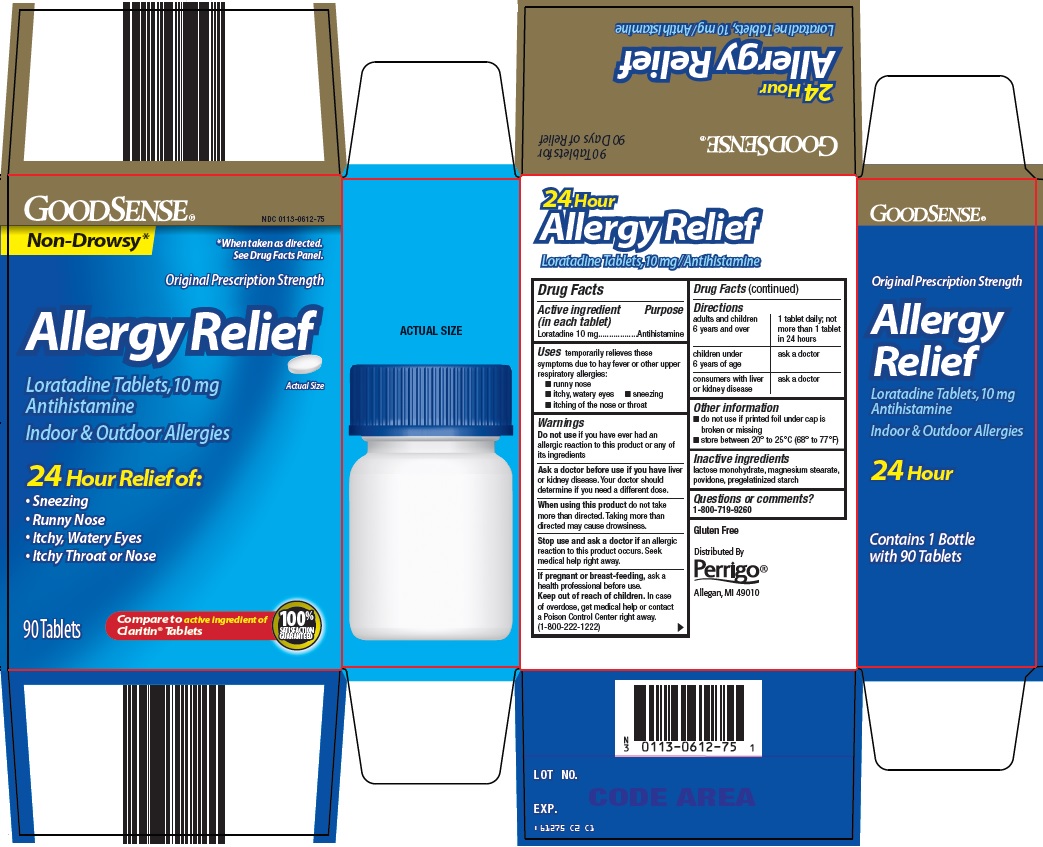
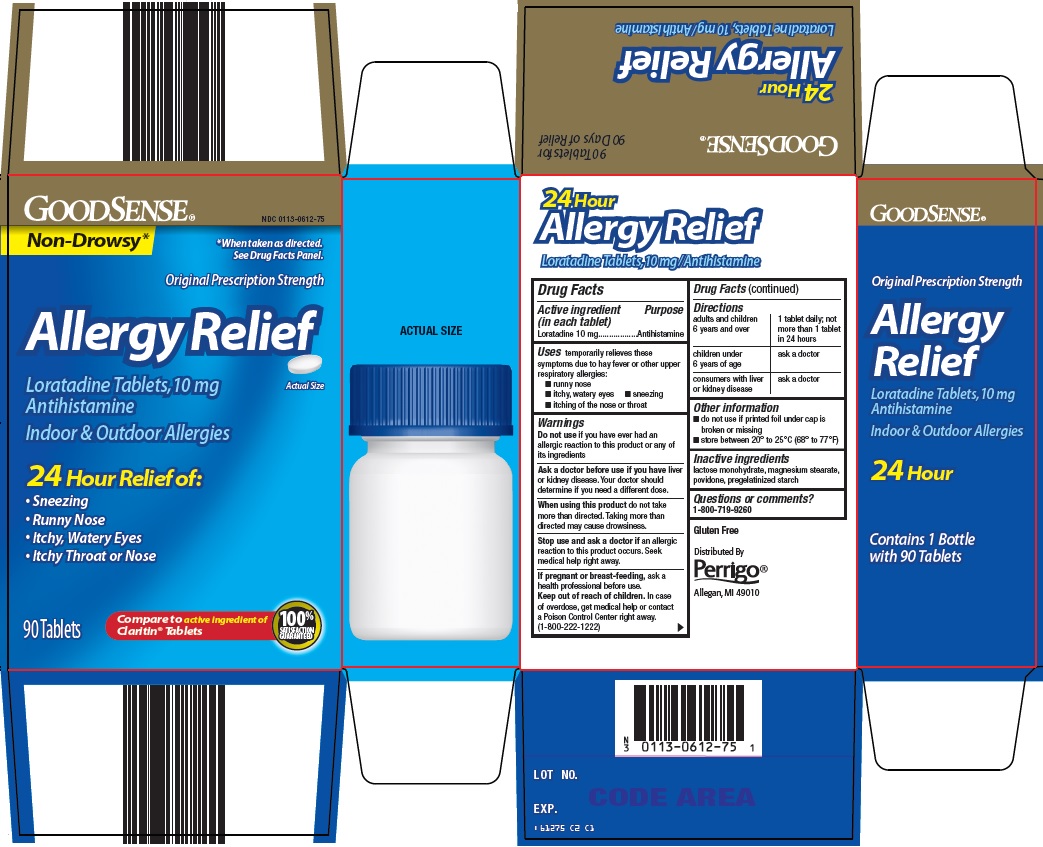
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Non-Drowsy*
*When taken as directed. See Drug Facts Panel.
Original Prescription Strength
Allergy Relief
Loratadine Tablets, 10 mg
Antihistamine
Actual Size
Indoor & Outdoor Allergies
24 Hour Relief of:
Sneezing
Runny Nose
Itchy, Watery Eyes
Itchy Throat or Nose
90 Tablets
Compare to active ingredient of Claritin® Tablets
100% SATISFACTION GUARANTEED
-
INGREDIENTS AND APPEARANCE
GOOD SENSE ALLERGY RELIEF
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0113-0612 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) Product Characteristics Color WHITE Score no score Shape OVAL Size 8mm Flavor Imprint Code L612 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0113-0612-39 30 in 1 CARTON 11/28/2005 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:0113-0612-46 10 in 1 CARTON 09/21/2004 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:0113-0612-60 20 in 1 CARTON 09/21/2004 3 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 4 NDC:0113-0612-65 1 in 1 CARTON 10/06/2004 4 30 in 1 BOTTLE; Type 0: Not a Combination Product 5 NDC:0113-0612-49 40 in 1 CARTON 05/28/2013 12/31/2019 5 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 6 NDC:0113-0612-88 1 in 1 CARTON 09/21/2004 6 365 in 1 BOTTLE; Type 0: Not a Combination Product 7 NDC:0113-0612-58 1 in 1 CARTON 09/21/2004 09/21/2004 7 40 in 1 BOTTLE; Type 0: Not a Combination Product 8 NDC:0113-0612-75 1 in 1 CARTON 06/06/2020 8 90 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076301 09/21/2004 Labeler - L. Perrigo Company (006013346)