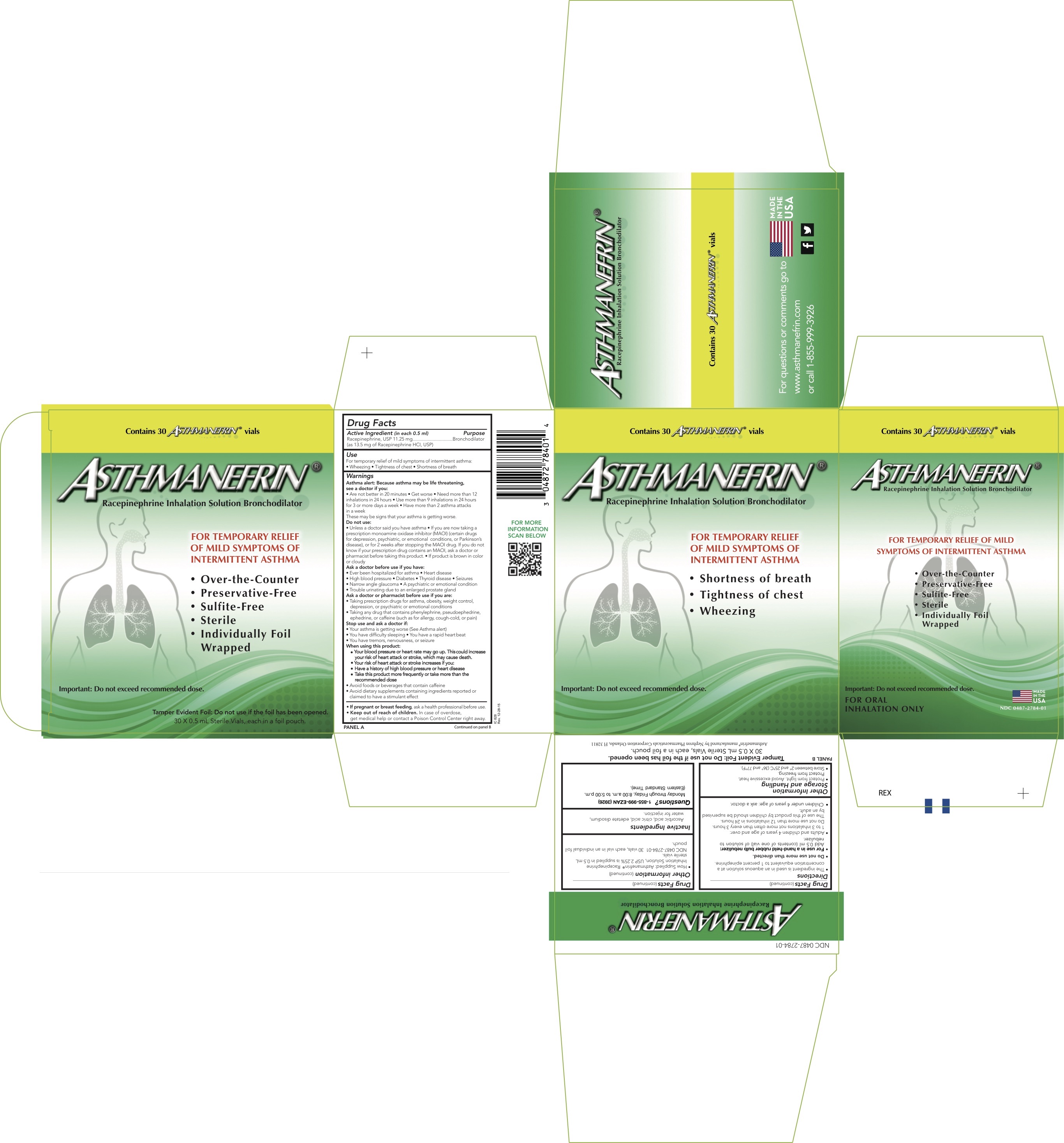
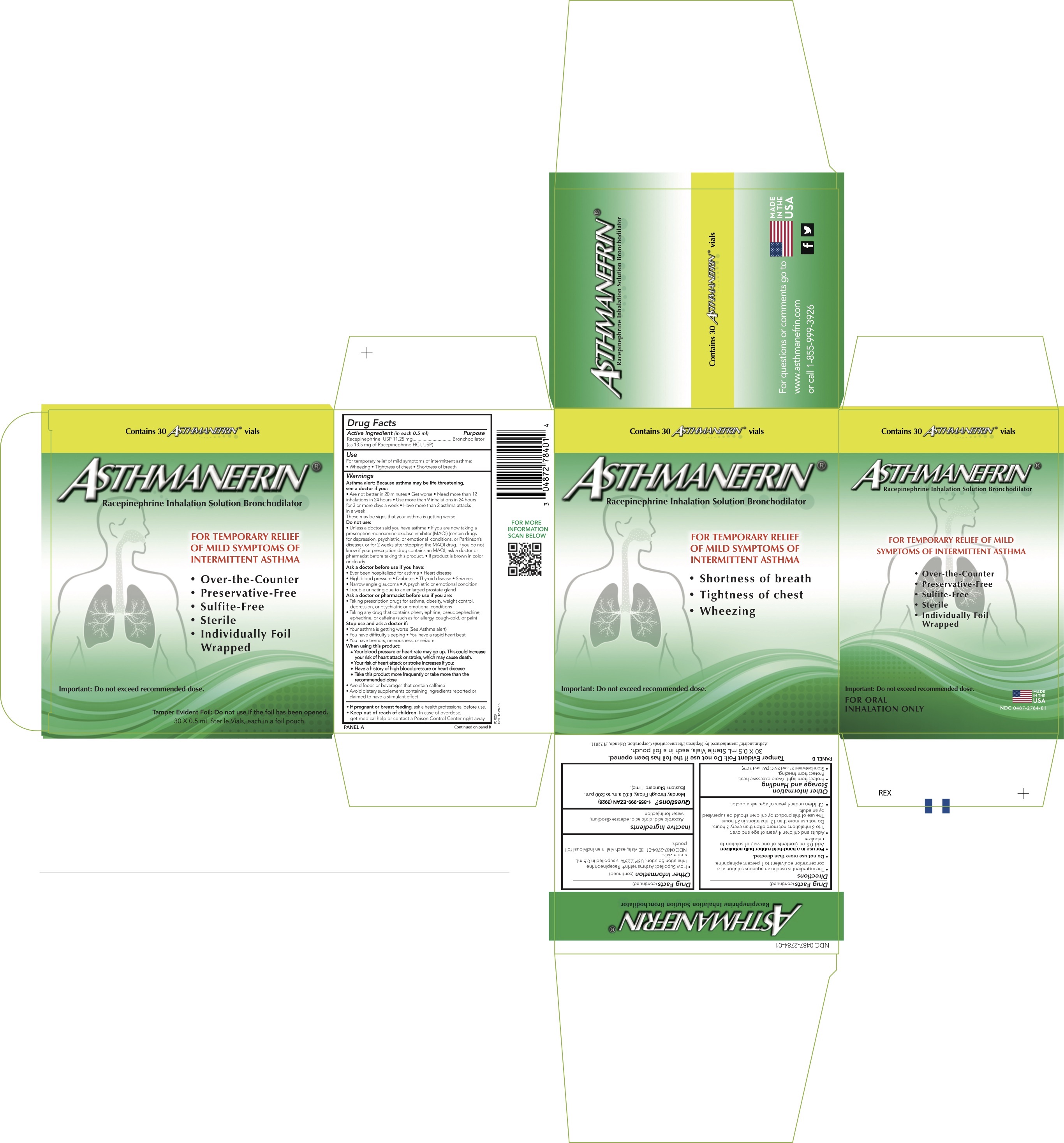
Label: ASTHMANEFRIN- racepinephrine hydrochloride solution
- NDC Code(s): 0487-2784-01
- Packager: Nephron Pharmaceuticals Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 11, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 0.5 ml)
- Purpose
- Uses
-
Warnings
Asthma alert: Because asthma may be life threatening, see a doctor if you:
- Are not better in 20 minutes
- Get worse
- Need more than 12 inhalations in 24 hours
- Use more than 9 inhalations in 24 hours for 3 or more days a week
- Have more than 2 asthma attacks in a week
These may be signs that your asthma is getting worse.
Do not use:
- Unless a doctor said you have asthma
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains a MAOI, ask a doctor or pharmacist before taking this product.
- If product is brown in color or cloudy
Ask a doctor before use if you have:
- Ever been hospitalized for asthma
- Heart disease
- High blood pressure
- Diabetes
- Thyroid disease
- Seizures
- Narrow angle glaucoma
- A psychiatric or emotional condition
- Trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are:
- Taking prescription drugs for asthma, obesity, weight control, depression, or psychiatric or emotional conditions.
- Taking any drug that contains phenylephrine, pseudoephedrine, ephedrine, or caffeine (such as for allergy, cough-cold, or pain).
Stop use and ask a doctor if:
- Your asthma is getting worse (See Asthma alert)
- You have difficulty sleeping
- You have a rapid heartbeat
- You have tremors, nervousness, or seizure
When using this product:
- Your blood pressure or heart rate may go up. This could increase your risk of heart attack or stroke, which may cause death.
-
Your risk of heart attack or stroke increases if you:
- Have a history of high blood pressure or heart disease
- Take this product more frequently or take more than the recommended dose.
- Avoid foods or beverages that contain caffeine
- Avoid dietary supplements containing ingredients reported or claimed to have a stimulant effect.
-
Directions
- The ingredient is used in an aqueous solution at a concentration equivalent to 1 percent epinephrine.
- Do not use more than directed
- For use in a hand-held rubber bulb nebulizer:
- Add 0.5 mL (contents of one vial) of solution to nebulizer.
- Adults and children 4 years of age and over:
1 to 3 inhalations not more often than every 3 hours.
Do not use more than 12 inhalations in 24 hours.
The use of this product by children should be supervised by an adult. - Children under 4 years of age: ask a doctor.
-
Other Information
Storage and Handling
- Protect from light. Avoid excessive heat. Protect from freezing.
- Store between 2°C and 25°C (36°F and 77°F).
- How Supplied: Asthmanefrin
® Racepinephrine Inhalation Solution, USP 2.25% is supplied in 0.5 mL sterile vials.
NDC 0487-2784-01 30 vials, each vial in an individual foil pouch.
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Carton
-
INGREDIENTS AND APPEARANCE
ASTHMANEFRIN
racepinephrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0487-2784 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RACEPINEPHRINE HYDROCHLORIDE (UNII: 336096P2WE) (RACEPINEPHRINE - UNII:GR0L9S3J0F) RACEPINEPHRINE 11.25 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0487-2784-01 30 in 1 CARTON 08/01/2012 1 1 in 1 POUCH 1 0.5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2012 Labeler - Nephron Pharmaceuticals Corporation (079160190) Registrant - Nephron SC, Inc. (079160190) Establishment Name Address ID/FEI Business Operations Nephron SC, Inc. 079160190 analysis(0487-2784) , pack(0487-2784) , manufacture(0487-2784) , label(0487-2784)