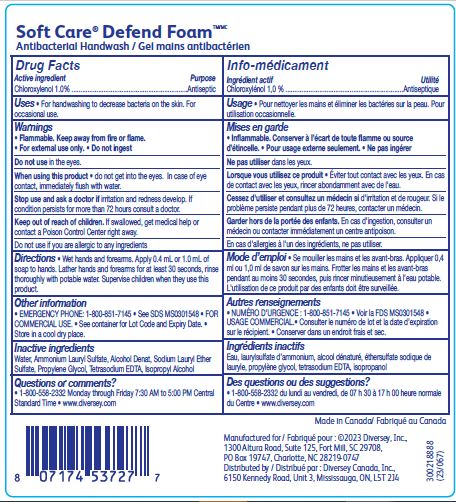
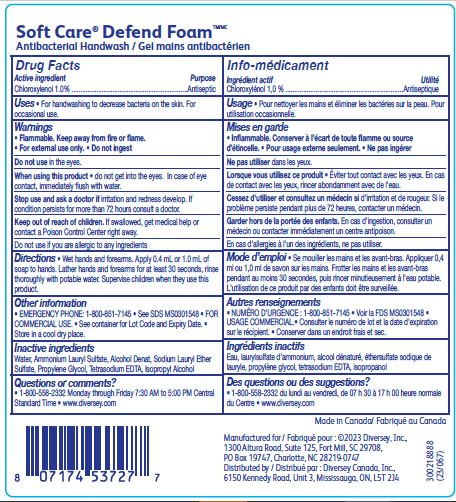
Label: SOFT CARE DEFEND FOAM ANTIBACTERIAL HANDWASH- chloroxylenol solution
- NDC Code(s): 64536-8308-2, 64536-8308-3
- Packager: Diversey, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 14, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- REFERENCES
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOFT CARE DEFEND FOAM ANTIBACTERIAL HANDWASH
chloroxylenol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64536-8308 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) ISOPROPYL ALCOHOL (UNII: ND2M416302) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64536-8308-2 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2016 12/22/2018 2 NDC:64536-8308-3 1200 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/29/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 07/01/2016 Labeler - Diversey, Inc. (018240817) Establishment Name Address ID/FEI Business Operations Diversey Canada, Inc. 249266974 manufacture(64536-8308)