Label: PEPCID COMPLETE- famotidine, calcium carbonate, and magnesium hydroxide tablet, chewable
-
Contains inactivated NDC Code(s)
NDC Code(s): 52904-948-03, 52904-948-07, 52904-948-20, 52904-948-25 - Packager: Select Corporation
- This is a repackaged label.
- Source NDC Code(s): 16837-246
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
-
Warnings
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- kidney disease
Ask a doctor or pharmacist before use if you are taking a prescription drug. Antacids and acid reducers may interact with certain prescription drugs.
- Directions
- Other information
-
Inactive ingredients
cellulose acetate, corn starch, crospovidone, D&C red no.7 calcium lake, dextrose excipient, FD&C blue no.1aluminum lake, FD&C red no.40 aluminum lake, flavors, gum arabic, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearrate, maltodextrin, mineral oil, modified starch, sucralose
- Questions or comments?
- SPL UNCLASSIFIED SECTION
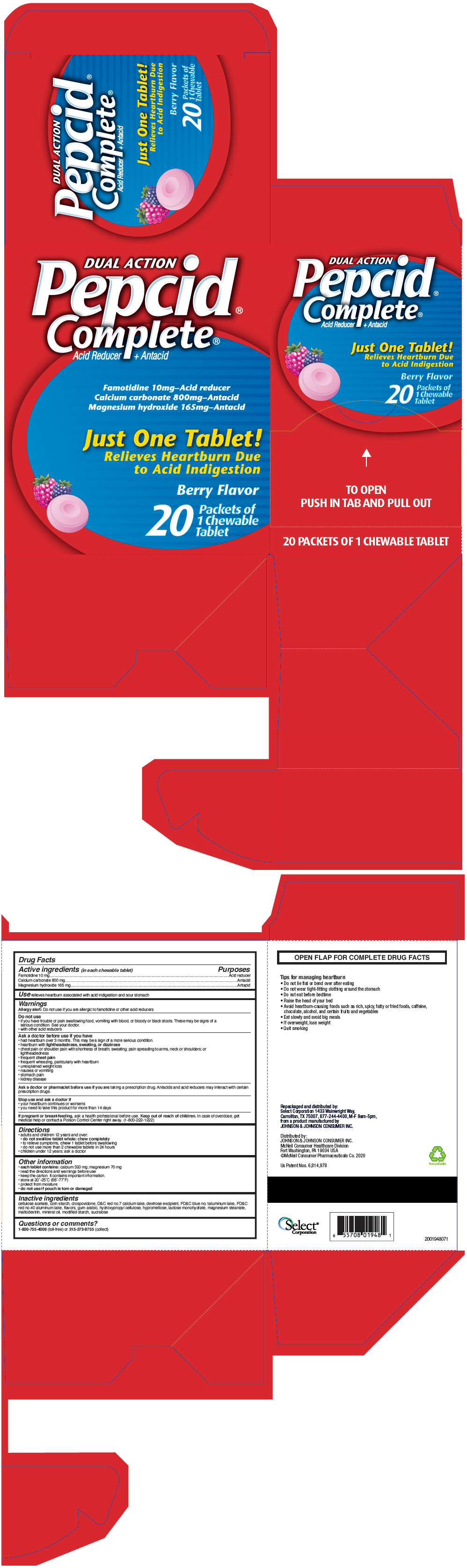
- PRINCIPAL DISPLAY PANEL - 20 Packet Carton
-
INGREDIENTS AND APPEARANCE
PEPCID COMPLETE
famotidine, calcium carbonate, and magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52904-948(NDC:16837-246) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength famotidine (UNII: 5QZO15J2Z8) (famotidine - UNII:5QZO15J2Z8) famotidine 10 mg calcium carbonate (UNII: H0G9379FGK) (carbonate ion - UNII:7UJQ5OPE7D) calcium carbonate 800 mg magnesium hydroxide (UNII: NBZ3QY004S) (hydroxide ion - UNII:9159UV381P) magnesium hydroxide 165 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) corn syrup (UNII: 9G5L16BK6N) crospovidone (120 .mu.m) (UNII: 68401960MK) dextrose, unspecified form (UNII: IY9XDZ35W2) FD&C yellow no. 5 (UNII: I753WB2F1M) aluminum oxide (UNII: LMI26O6933) FD&C yellow no. 6 (UNII: H77VEI93A8) acacia (UNII: 5C5403N26O) hydroxypropyl cellulose, unspecified (UNII: 9XZ8H6N6OH) hypromellose, unspecified (UNII: 3NXW29V3WO) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) maltodextrin (UNII: 7CVR7L4A2D) mineral oil (UNII: T5L8T28FGP) sucralose (UNII: 96K6UQ3ZD4) triacetin (UNII: XHX3C3X673) cellulose acetate (UNII: 3J2P07GVB6) Product Characteristics Color YELLOW Score no score Shape ROUND Size 17mm Flavor FRUIT (tropical fruit flavor) Imprint Code P Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52904-948-03 1 in 1 BLISTER PACK 01/01/2009 1 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:52904-948-07 2 in 1 BLISTER PACK 01/01/2009 2 1 in 1 POUCH; Type 0: Not a Combination Product 3 NDC:52904-948-20 20 in 1 CARTON 01/01/2009 3 1 in 1 POUCH; Type 0: Not a Combination Product 4 NDC:52904-948-25 25 in 1 CARTON 01/01/2009 4 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020958 01/01/2009 Labeler - Select Corporation (053805599)