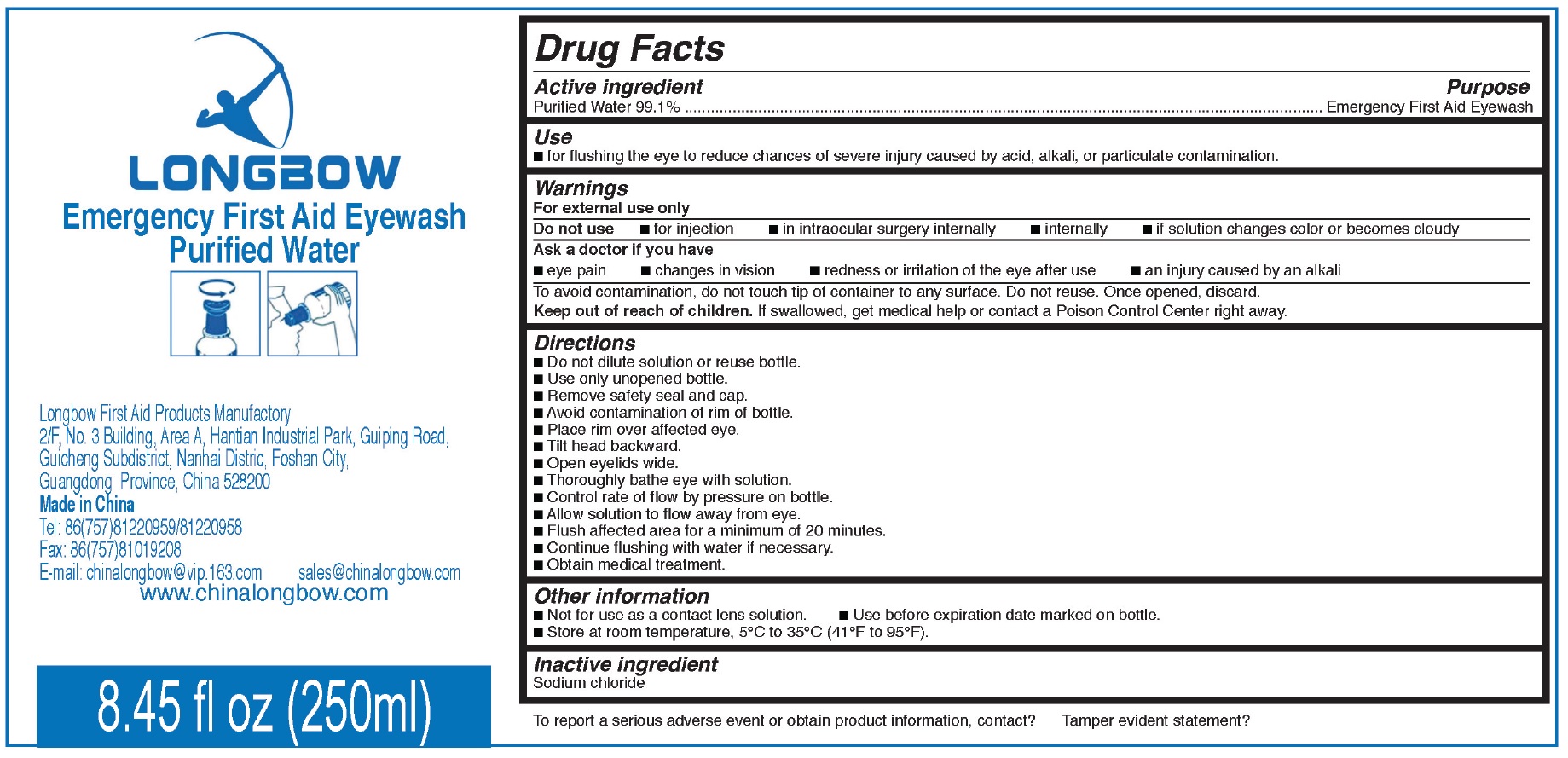
Label: EMERGENCY FIRST AID EYEWASH PURIFIED WATER- water liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 70746-001-01, 70746-001-02 - Packager: Longbow First Aid Products Manufactory
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 8, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
-
Warnings
For external use only.
Do not use
- for injection
- in intraocular surgery internally
- internally
- if solution changes color and become cloudy
Ask a doctor if you have
- eye pain
- changes in vision
- redness or irritation occurs of the eye after use
- an injury caused by an alkali
-
Directions
- Do not dilute solution or reuse bottle.
- Use only unopened bottle.
- Remove safely seal and cap
- Avoid contamination of rim of bottle.
- Place rim over affected eye.
- Tilt head backward.
- Open eyelids wide.
- Thoroughly bathe eye with solution.
- Control rate of flow by pressure on bottle.
- Allow solution to flow away from eye.
- Flush affected area for a minumum of 20 minutes.
- Continue flushing with water if necessary.
- Obtain medical treatment.
- Other information
- Inactive ingredient
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
EMERGENCY FIRST AID EYEWASH PURIFIED WATER
water liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70746-001 Route of Administration IRRIGATION Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 999.1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70746-001-01 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/07/2016 2 NDC:70746-001-02 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/07/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 12/07/2016 Labeler - Longbow First Aid Products Manufactory (529128690)