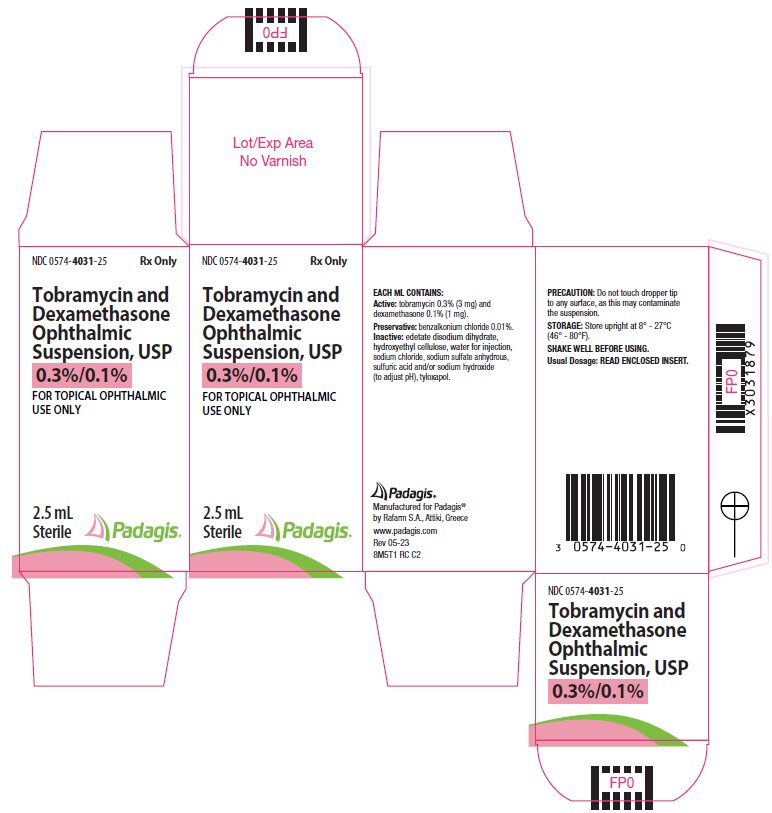
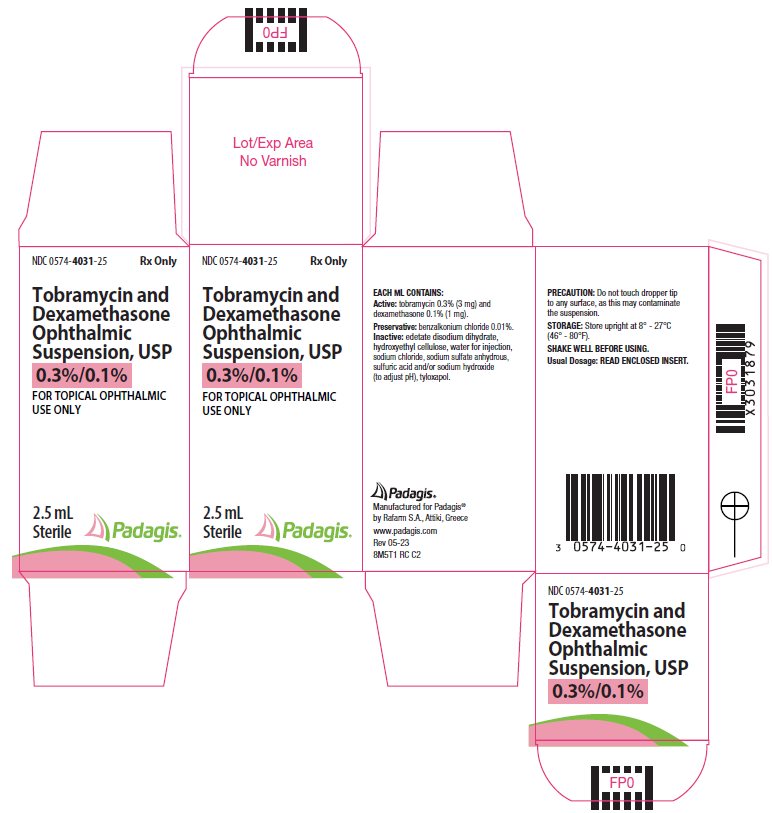
Label: TOBRAMYCIN AND DEXAMETHASONE suspension/ drops
- NDC Code(s): 0574-4031-05, 0574-4031-10, 0574-4031-25
- Packager: Padagis US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
DESCRIPTION: Tobramycin and dexamethasone ophthalmic suspension USP is a sterile, multiple dose antibiotic and steroid combination for topical ophthalmic use with a pH of approximately 5.5 and an osmolality of approximately 300 mOsm/kg.
The chemical structures for tobramycin and dexamethasone are presented below:
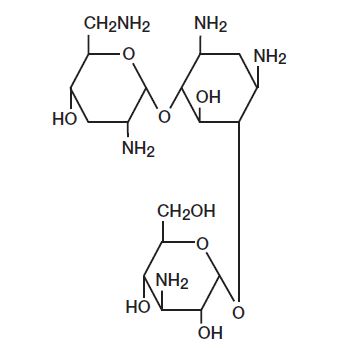
Tobramycin
Empirical Formula: C18H37N5O9
Chemical Name: O-3-Amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine
MW: 467.52
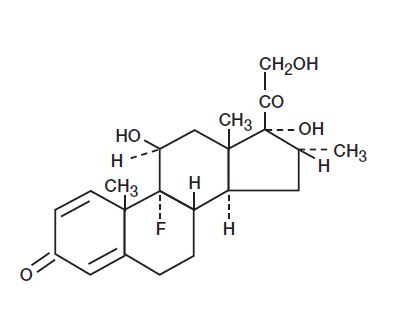
Dexamethasone
Empirical Formula: C22H29FO5
Chemical Name: 9-Fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione
MW: 392.47
Each mL of Tobramycin and dexamethasone ophthalmic suspension contains: Actives: tobramycin 0.3% (3 mg) and dexamethasone 0.1% (1 mg). Preservative: benzalkonium chloride 0.01%. Inactives: edetate disodium dihydrate, hydroxyethyl cellulose, sodium chloride, sodium sulfate anhydrous, sulfuric acid and/or sodium hydroxide (to adjust pH), tyloxapol, and water for injection.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY: Corticoids suppress the inflammatory response to a variety of agents and they probably delay or slow healing. Since corticoids may inhibit the body's defense mechanism against infection, a concomitant antimicrobial drug may be used when this inhibition is considered to be clinically significant. Dexamethasone is a potent corticoid.
The antibiotic component in the combination (tobramycin) is included to provide action against susceptible organisms. In vitro studies have demonstrated that tobramycin is active against susceptible strains of the following microorganisms:
Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
No data are available on the extent of systemic absorption from tobramycin and dexamethasone ophthalmic suspension; however, it is known that some systemic absorption can occur with ocularly applied drugs.
-
INDICATIONS & USAGE
INDICATIONS AND USAGE: Tobramycin and dexamethasone ophthalmic suspension is indicated for steroid-responsive inflammatory ocular conditions for which a corticosteroid is indicated and where superficial bacterial ocular infection or a risk of bacterial ocular infection exists.
Ocular steroids are indicated in inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe where the inherent risk of steroid use in certain infective conjunctivitides is accepted to obtain a diminution in edema and inflammation. They are also indicated in chronic anterior uveitis and corneal injury from chemical, radiation or thermal burns, or penetration of foreign bodies.
The use of a combination drug with an anti-infective component is indicated where the risk of superficial ocular infection is high or where there is an expectation that potentially dangerous numbers of bacteria will be present in the eye.
The particular anti-infective drug in this product is active against the following common bacterial eye pathogens:
Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species.
- CONTRAINDICATIONS
-
WARNINGS
WARNINGS: FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION INTO THE EYE. Sensitivity to topically applied aminoglycosides may occur in some patients. Severity of hypersensitivity reactions may vary from local effects to generalized reactions such as erythema, itching, urticaria, skin rash, anaphylaxis, anaphylactoid reaction, or bullous reactions. If a sensitivity reaction does occur, discontinue use.
Prolonged use of steroids may result in glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Intraocular pressure (IOP) should be routinely monitored even though it may be difficult in pediatric patients and uncooperative patients. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions and parasitic infections of the eye, steroids may mask infection or enhance existing infection.
In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical steroids.
-
PRECAUTIONS:
General: The possibility of fungal infections of the cornea should be considered after long-term steroid dosing. As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated. When multiple prescriptions are required, or whenever clinical judgement dictates, the patient should be examined with the aid of magnification, such as slit lamp biomicroscopy and, where appropriate, fluorescein staining.
Cross-sensitivity to other aminoglycoside antibiotics may occur; if hypersensitivity develops with this product, discontinue use and institute appropriate therapy.
Information for Patients: Do not touch dropper tip to any surface, as this may contaminate the contents. Contact lenses should not be worn during the use of this product.
Carcinogenesis, Mutagenesis, Impairment of Fertility: No studies have been conducted to evaluate the carcinogenic or mutagenic potential. No impairment of fertility was noted in studies of subcutaneous tobramycin in rats at doses of 50 and 100 mg/kg/day.
Pregnancy: Corticosteroids have been found to be teratogenic in animal studies. Ocular administration of 0.1% dexamethasone resulted in 15.6% and 32.3% incidence of fetal anomalies in two groups of pregnant rabbits. Fetal growth retardation and increased mortality rates have been observed in rats with chronic dexamethasone therapy. Reproduction studies have been performed in rats and rabbits with tobramycin at doses up to 100 mg/kg/day parenterally and have revealed no evidence of impaired fertility or harm to the fetus.
There are no adequate and well-controlled studies in pregnant women. However, prolonged or repeated corticoid use during pregnancy has been associated with an increased risk of intra-uterine growth retardation. Tobramycin and dexamethasone ophthalmic suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be observed carefully for signs of hypoadrenalism.
Nursing Mothers: Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when tobramycin and dexamethasone ophthalmic suspension is administered to a nursing woman.
-
ADVERSE REACTIONS
ADVERSE REACTIONS: Adverse reactions have occurred with steroid/anti-infective combination drugs which can be attributed to the steroid component, the anti-infective component, or the combination. Exact incidence figures are not available.
The most frequent adverse reactions to topical ocular tobramycin (tobramycin ophthalmic solution) are hypersensitivity and localized ocular toxicity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than 4% of patients.
The reactions due to the steroid component are: elevation of IOP with possible development of glaucoma, and infrequent optic nerve damage; posterior subcapsular cataract formation; and delayed wound healing.
Secondary Infection: The development of secondary infection has occurred after use of combinations containing steroids and antimicrobials. Fungal infections of the cornea are particularly prone to develop coincidentally with long-term applications of steroids. The possibility of fungal invasion must be considered in any persistent corneal ulceration where steroid treatment has been used. Secondary bacterial ocular infection following suppression of host responses also occurs.
- Postmarketing Experience: Additional adverse reactions identified from post-marketing use include anaphylactic reaction, erythema multiforme.
The following additional adverse reactions have been reported with the individual components listed below:
Dexamethasone: Cushing's syndrome and adrenal suppression may occur after use of dexamethasone in excess of the listed dosing instructions in predisposed patients, including children and patients treated with CYP3A4 inhibitors.
Aminoglycosides: Neurotoxicity, ototoxicity, and nephrotoxicity have occurred in patients receiving systemic aminoglycoside therapy. Aminoglycosides may aggravate muscle weakness in patients with known or suspected neuromuscular disorders, such as myasthenia gravis or Parkinson's disease, because of their potential effect on neuromuscular function.
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION: One or two drops instilled into the conjunctival sac(s) every four to six hours. During the initial 24 to 48 hours, the dosage may be increased to one or two drops every two (2) hours. Frequency should be decreased gradually as warranted by improvement in clinical signs. Care should be taken not to discontinue therapy prematurely.
Not more than 20 mL should be prescribed initially and the prescription should not be refilled without further evaluation as outlined in PRECAUTIONS above.
-
HOW SUPPLIED
HOW SUPPLIED: Tobramycin and dexamethasone ophthalmic suspension, USP is sterile ophthalmic suspension supplied as a 2.5 mL, 5 mL, or 10 mL suspension in a 5 mL, 10 mL or 10 mL natural polyethylene bottle respectively with a natural polyethylene controlled drop tip and a white polypropylene cap.
NDC 0574-4031-25: 2.5 mL
NDC 0574-4031-05: 5 mL
NDC 0574-4031-10: 10 mL
STORAGE: Store at 8°C to 27°C (46°F to 80°F). Store suspension upright and shake well before using. After opening, tobramycin and dexamethasone ophthalmic suspension can be used until the expiration date on the bottle.
Rx Only
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
NDC 0574-4031-25
Rx Only
Tobramycin and Dexamethasone Ophthalmic Suspension, USP
0.3%/0.1%FOR TOPICAL OPHTHALMIC USE ONLY
2.5 mL
Sterile
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.
-
INGREDIENTS AND APPEARANCE
TOBRAMYCIN AND DEXAMETHASONE
tobramycin and dexamethasone suspension/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0574-4031 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOBRAMYCIN (UNII: VZ8RRZ51VK) (TOBRAMYCIN - UNII:VZ8RRZ51VK) TOBRAMYCIN 3 mg in 1 mL DEXAMETHASONE (UNII: 7S5I7G3JQL) (DEXAMETHASONE - UNII:7S5I7G3JQL) DEXAMETHASONE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM SULFATE (UNII: 0YPR65R21J) SULFURIC ACID (UNII: O40UQP6WCF) SODIUM HYDROXIDE (UNII: 55X04QC32I) TYLOXAPOL (UNII: Y27PUL9H56) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0574-4031-25 2.5 mL in 1 BOTTLE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 03/17/2022 2 NDC:0574-4031-05 5 mL in 1 BOTTLE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 03/17/2022 3 NDC:0574-4031-10 10 mL in 1 BOTTLE, PLASTIC; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 03/17/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212715 03/17/2022 Labeler - Padagis US LLC (967694121)