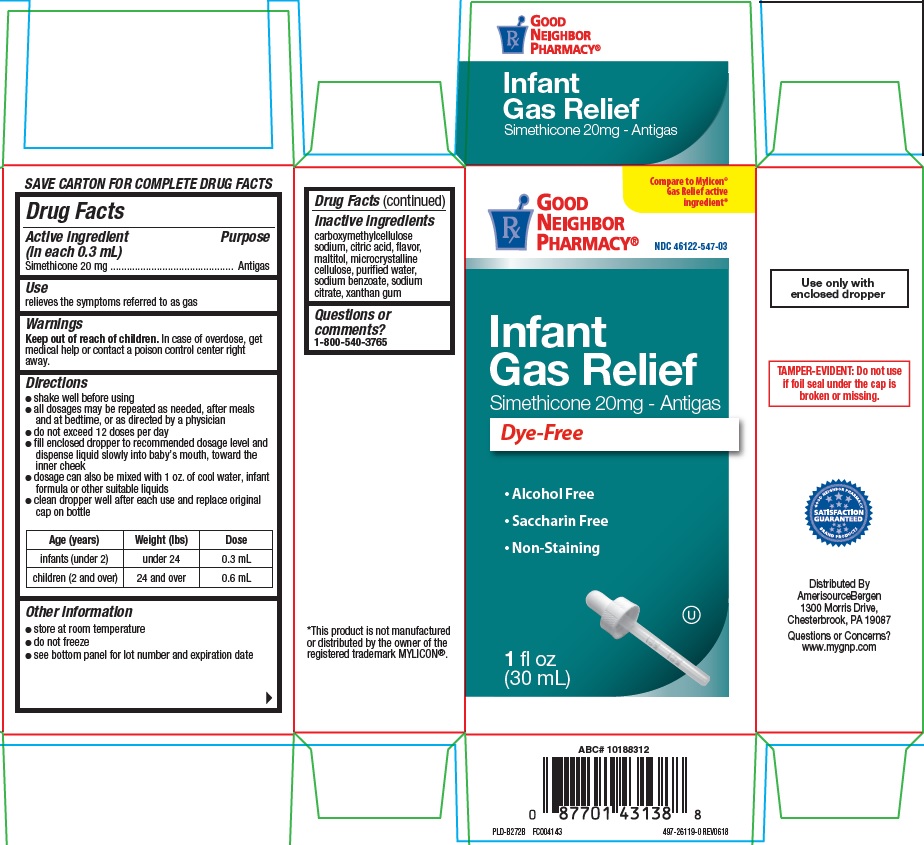
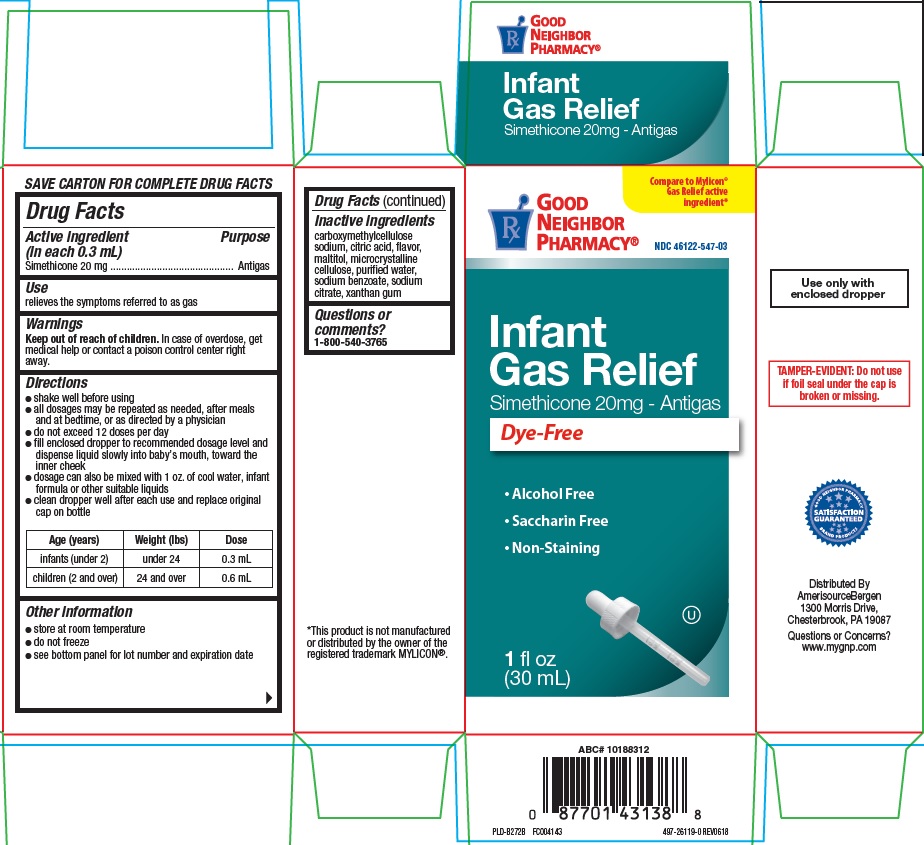
Label: INFANT GAS RELIEF- simethicone suspension/ drops
- NDC Code(s): 46122-547-03
- Packager: AMERISOURCEBERGEN DRUG CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 0.3 mL)
- Purpose
- Use
- Warnings
-
Directions
- shake well before using
- all dosages may be repeated as needed, after meals and at bedtime, or as directed by a physician
- do not exceed 12 doses per day
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby’s mouth, toward inner cheek
- dosage can also be mixed with 1 oz. of cool water, infan formula or other suitable liquids
- clean dropper well after each use and replace original cap on bottle
Age (years) Weight (lbs) Dose infants (under 2) under 24 0.3 mL children (over 2) 24 and over 0.6 mL - Other information
- Inactive ingredients
- package Label
-
INGREDIENTS AND APPEARANCE
INFANT GAS RELIEF
simethicone suspension/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:46122-547 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MALTITOL (UNII: D65DG142WK) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color white Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46122-547-03 1 in 1 CARTON 11/16/2018 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 06/01/2018 Labeler - AMERISOURCEBERGEN DRUG CORPORATION (007914906) Registrant - GCP Laboratories (965480861) Establishment Name Address ID/FEI Business Operations GCP Laboratories 965480861 manufacture(46122-547)