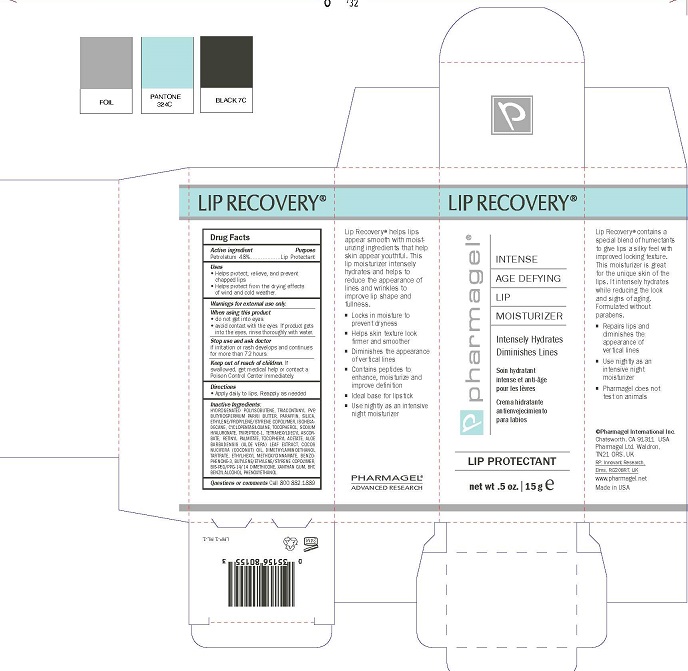
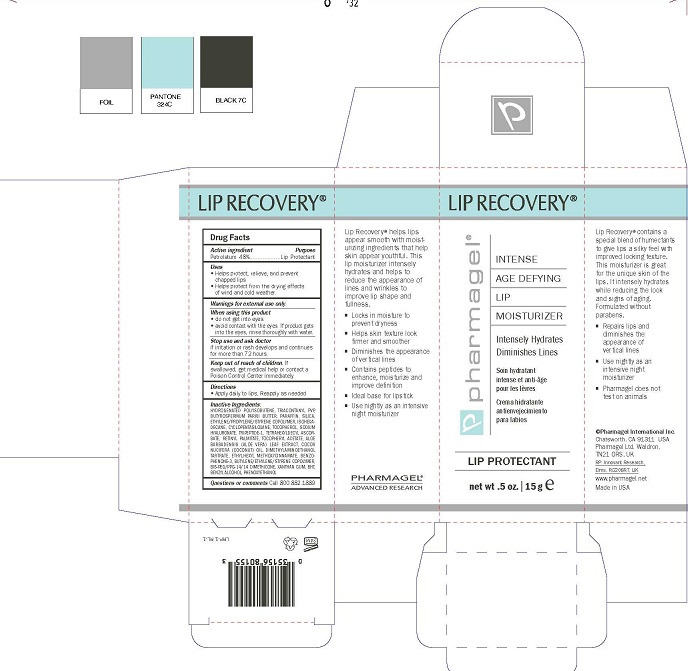
Label: LIP RECOVERY- petrolatum ointment
- NDC Code(s): 67879-307-11, 67879-307-51
- Packager: PHARMAGEL INTERNATIONAL INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- STOP USE AND ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- Directions:
-
INACTIVE INGREDIENTS:
HYDROGENATED POLYISOBUTENE, TRIACONTANYL PVP, BUTYROSPERMUM PARKII BUTTER, PARAFFIN, SILICA, ETHYLENE/PROPYLENE/STYRENE COPOLYMER, ISOHEXA- DECANE, CYCLOPENTASILOXANE, TOCOPHEROL, SODIUM HYALURONATE, TRIPEPTIDE-1, TETRAHEXYLDECYL ASCOR- BATE, RETINYL PALMITATE, TOCOPHERYL ACETATE, ALOE BARBADENSIS (ALOE VERA) LEAF EXTRACT, COCOS NUCIFERA (COCONUT) OIL, DIMETHYLAMINOETHANOL TARTRATE, ETHYLHEXYL METHOXYCINNAMATE, BENZO- PHENONE-3, BUTYLENE/ETHYLENE/STYRENE COPOLYMER, BIS-PEG/PPG-14/14 DIMETHICONE, XANTHAN GUM, BHT, BENZYL ALCOHOL,PHENOXYETHANOL
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIP RECOVERY
petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67879-307 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 48 g in 100 mL Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYBUTENE (1300 MW) (UNII: 7D1YQ9Y5EZ) TRICONTANYL POVIDONE (UNII: N0SS3Q238D) SHEA BUTTER (UNII: K49155WL9Y) PARAFFIN (UNII: I9O0E3H2ZE) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLENE (UNII: 91GW059KN7) PROPYLENE (UNII: AUG1H506LY) STYRENE (UNII: 44LJ2U959V) ISOHEXADECANE (UNII: 918X1OUF1E) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) TOCOPHEROL (UNII: R0ZB2556P8) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PREZATIDE (UNII: 39TG2H631E) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) COCONUT OIL (UNII: Q9L0O73W7L) DEANOL BITARTRATE (UNII: D240J05W14) OCTINOXATE (UNII: 4Y5P7MUD51) OXYBENZONE (UNII: 95OOS7VE0Y) 1-BUTENE (UNII: LY001N554L) BIS-PEG/PPG-14/14 DIMETHICONE (UNII: X2I70H0QJE) XANTHAN GUM (UNII: TTV12P4NEE) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BENZYL ALCOHOL (UNII: LKG8494WBH) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67879-307-51 1 in 1 BOX 06/13/2016 1 NDC:67879-307-11 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/18/2015 Labeler - PHARMAGEL INTERNATIONAL INC (603215182)