Label: ALLERGY RELIEF- loratadine capsule, liquid filled
- NDC Code(s): 11673-986-07, 11673-986-30
- Packager: TARGET CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 7, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
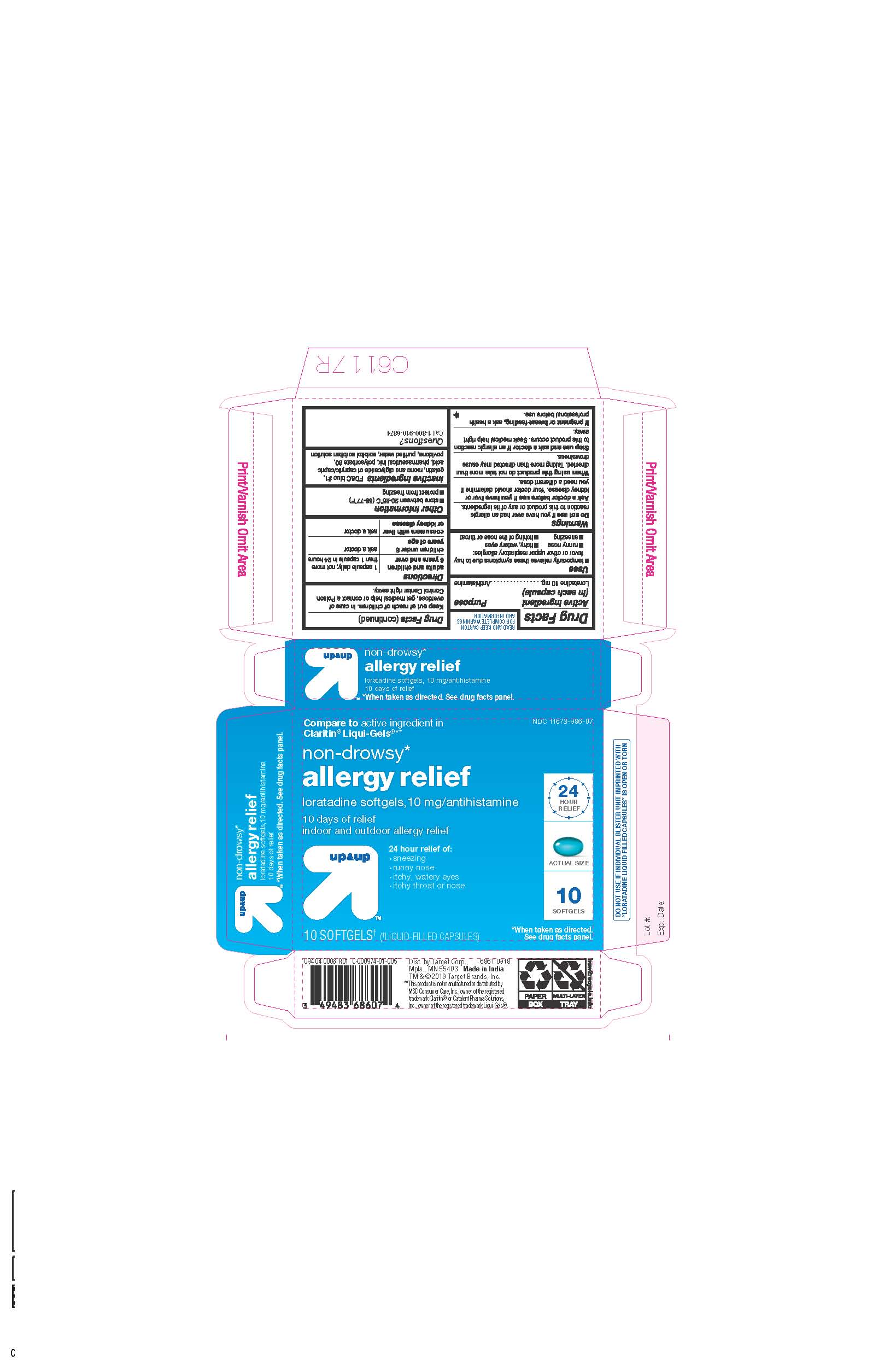
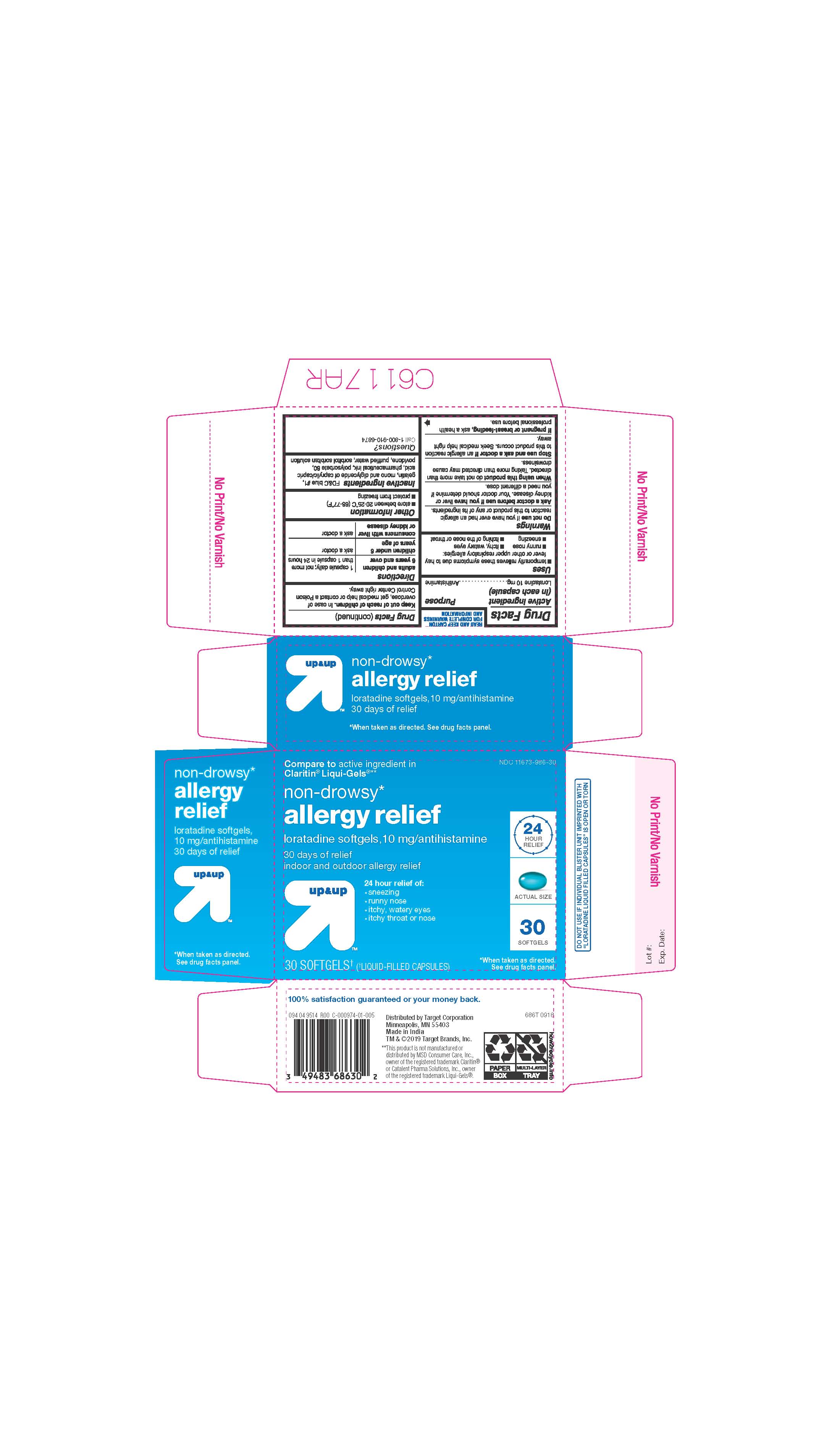
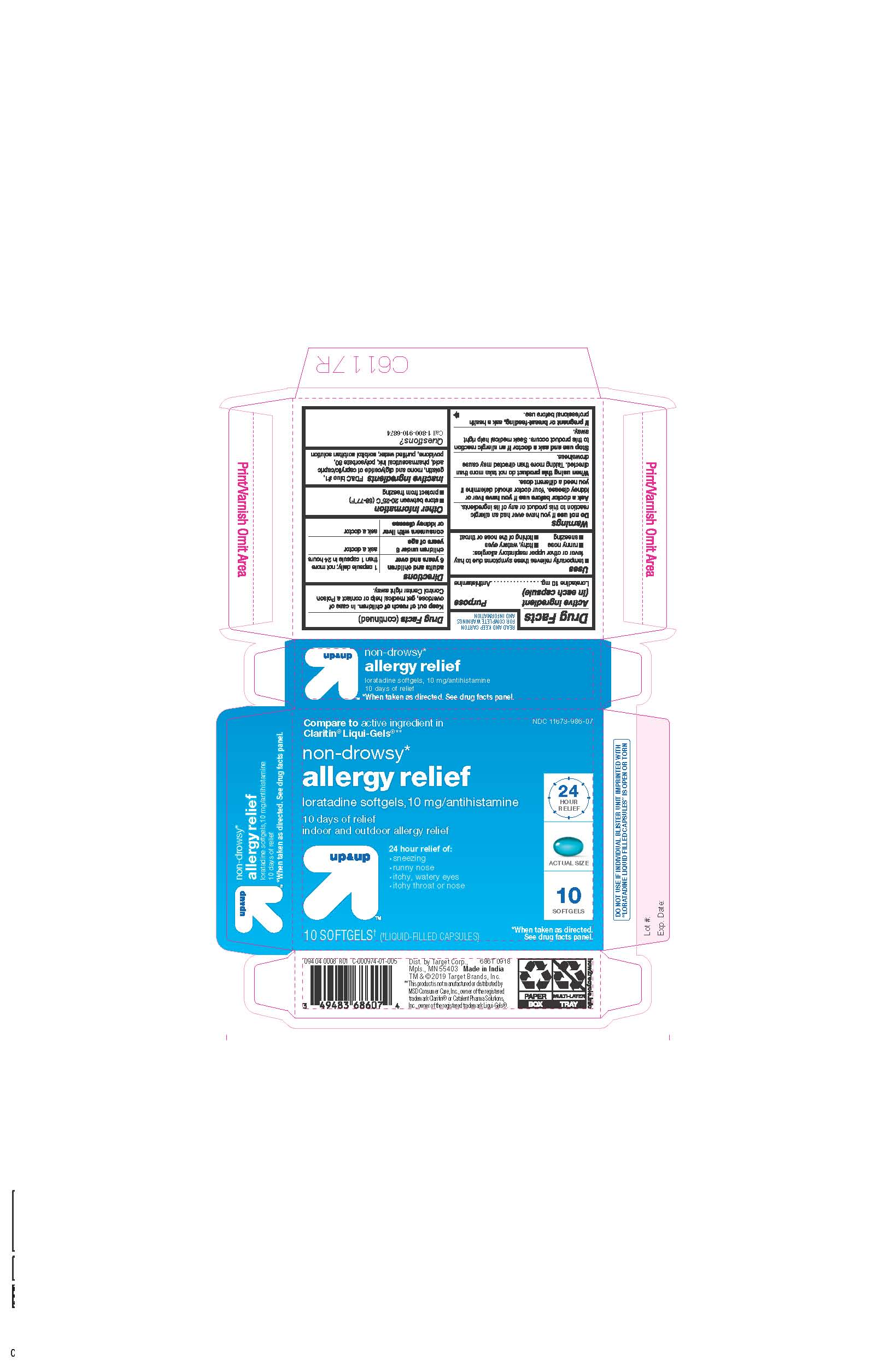
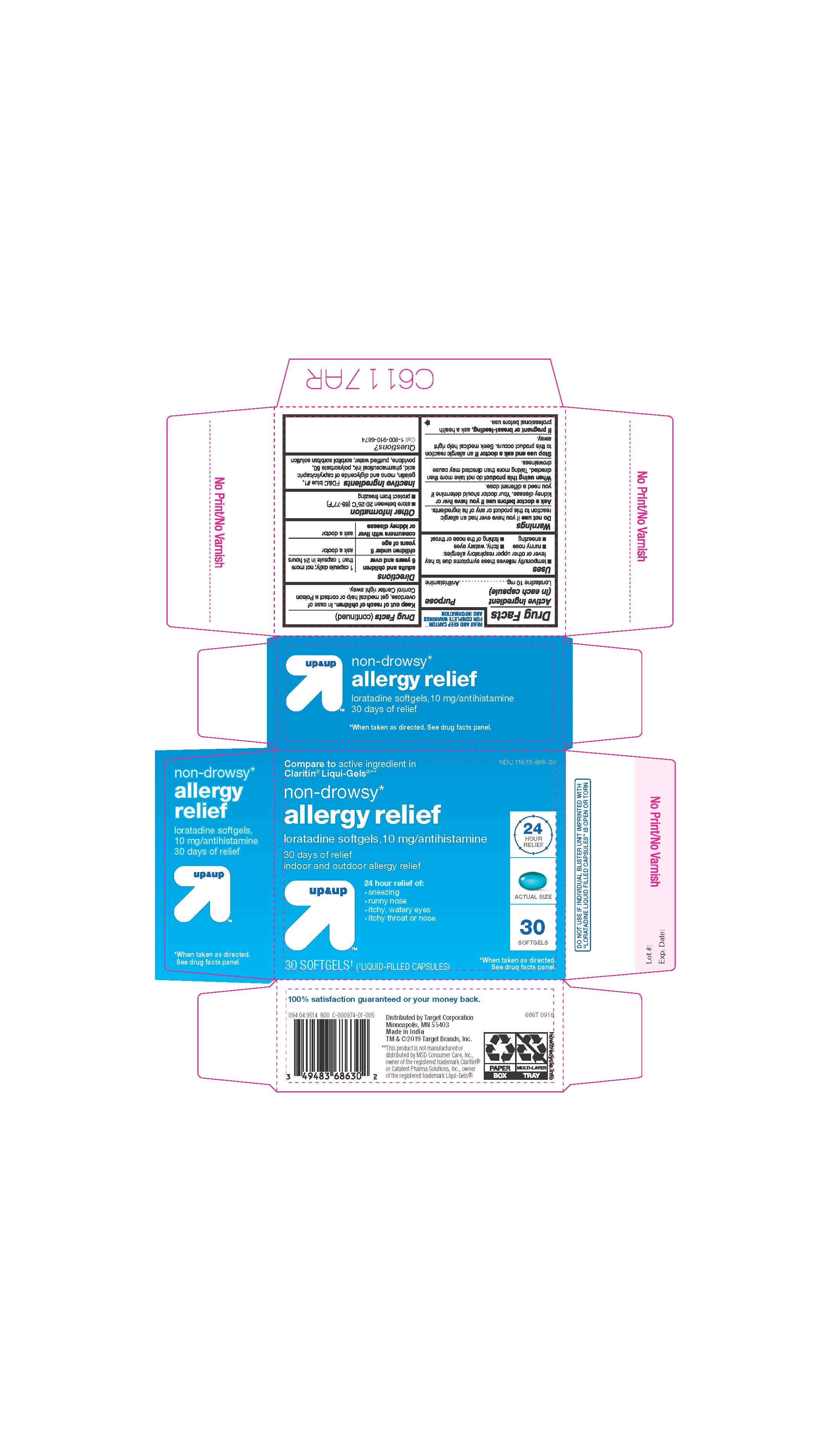
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
DO NOT USE IF YOU HAVE EVER HAD AN ALLERGIC REACTION TO THIS PRODUCT OR ANY OF ITS INGREDIENTS.
WHEN USING THIS PRODUCT DO NOT TAKE MORE THAN DIRECTED. TAKING MORE THAN DIRECTED MAY CAUSE DROWSINESS.
STOP USE AND ASK A DOCTOR IF AN ALLERGIC REACTION TO THIS PRODUCT OCCURS. SEEK MEDICAL HELP RIGHT AWAY.
IF PREGNANT OR BREAST FEEDING, ASK A HEALTH PROFESSIONAL BEFORE USE.
- INDICATIONS AND USAGE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- 10 CT BLISTER
- 30 COUNT BLISTER
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
loratadine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-986 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) CAPRYLIC/CAPRIC MONO/DI-GLYCERIDES (UNII: U72Q2I8C85) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color blue ((light blue)) Score no score Shape OVAL Size 3mm Flavor Imprint Code 21 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-986-07 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/26/2019 2 NDC:11673-986-30 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 04/26/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206214 04/10/2019 Labeler - TARGET CORPORATION (006961700) Registrant - TIME CAP LABORATORIES, INC. (037052099) Establishment Name Address ID/FEI Business Operations MARKSANS PHARMA LTD 925822975 manufacture(11673-986)