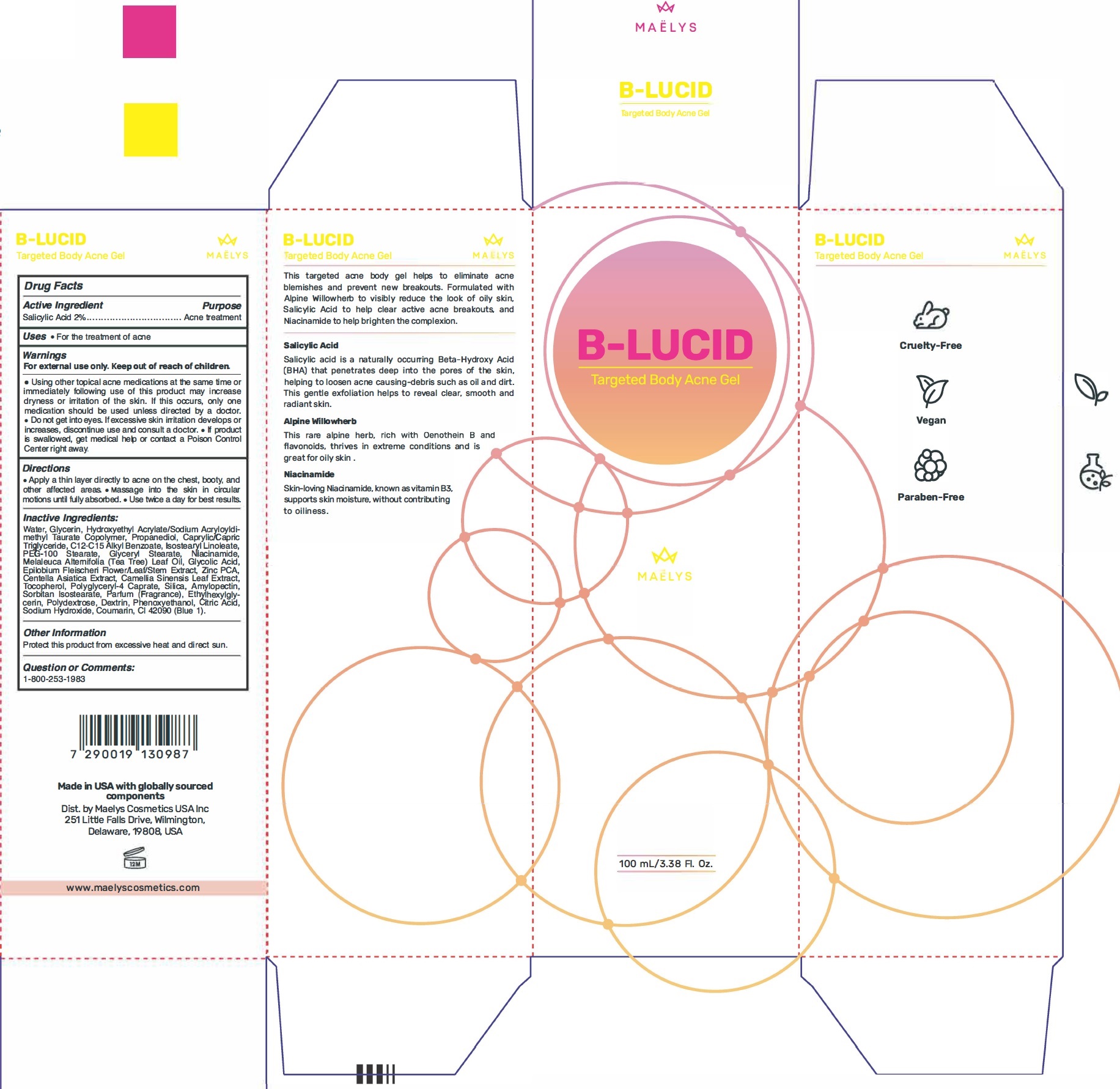
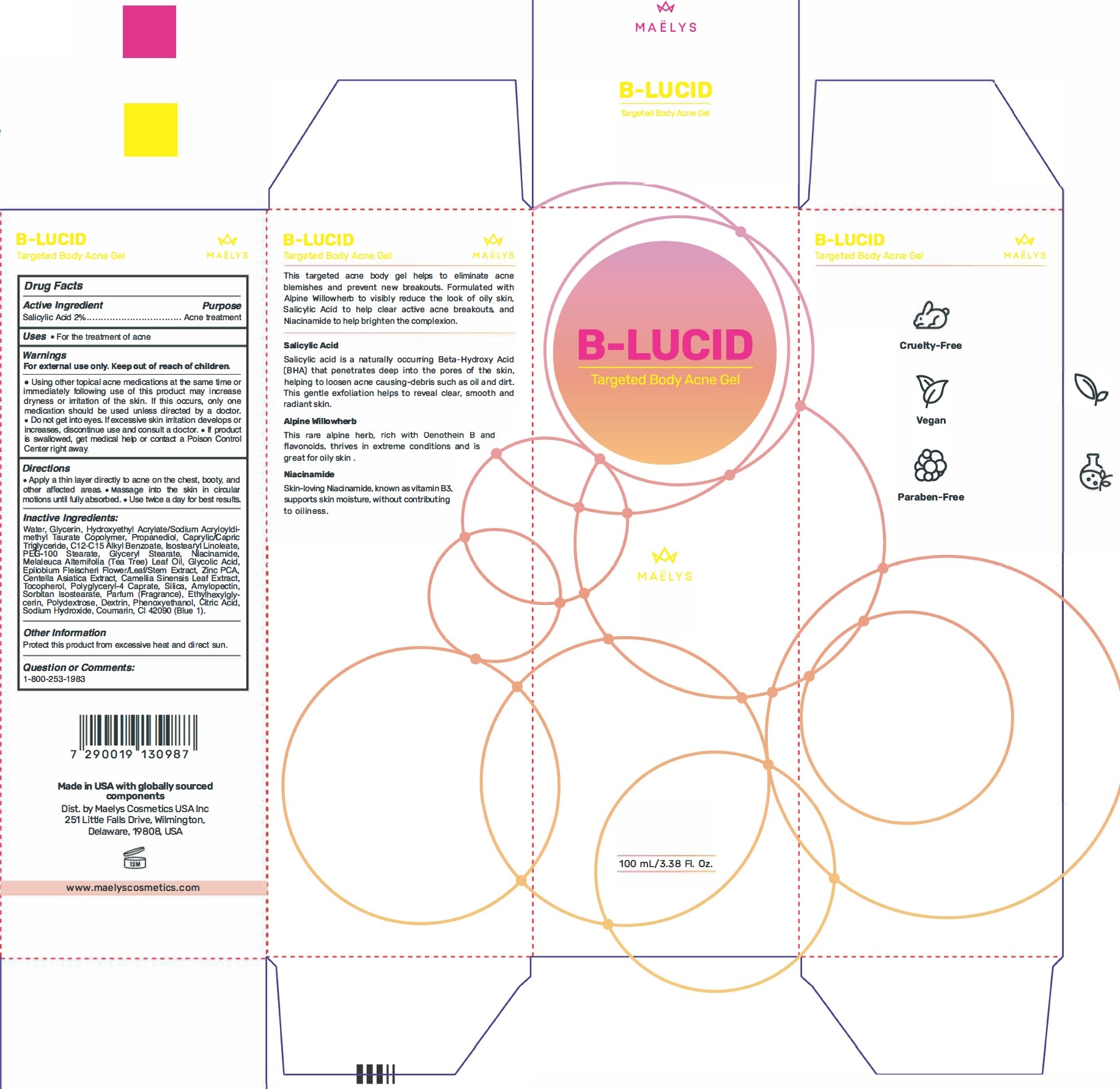
Label: B-LUCID TARGETED BODY ACNE GEL- salicylic acid gel
- NDC Code(s): 83010-010-00
- Packager: Maelys Cosmetics, LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
-
Warnings
For external use only.
Keep out of reach of children.
• Using other topical acne medications at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor. • Do not get into eyes. tt excessive skin irritation develops or increases, discontinue use and consult a doctor. • If product is swallowed, get medical help or contact a Poison Control Center right away.
- Directions
-
Inactive Ingredients:
Water, Glycerin, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Propanediol, Caprylic/Capric Triglyceride, C12-C15 Alkyl Benzoate, Isostearyl Linoleate, PEG-100 Stearate, Glyceryl Stearate, Niacinamide, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Glycolic Acid, Epilobium Fleischeri Flower/Leaf/Stem Extract, Zinc PCA, Centella Asiatica Extract, Camellia Sinensis Leaf Extract, Tocopherol, Polyglyceryl-4 Caprate, Silica, Amylopectin, Sorbitan Isostearate, Parfum (Fragrance), Ethylhexylglycerin, Polydextrose, Dextrin, Phenoxyethanol, Citric Acid, Sodium Hydroxide, Coumarin, CI 42090 (Blue 1).
- Other Information
- Question or Comments:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
B-LUCID TARGETED BODY ACNE GEL
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83010-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PROPANEDIOL (UNII: 5965N8W85T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ISOSTEARYL LINOLEATE (UNII: 4778M3HR0N) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) NIACINAMIDE (UNII: 25X51I8RD4) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) GLYCOLIC ACID (UNII: 0WT12SX38S) ZINC PIDOLATE (UNII: C32PQ86DH4) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TOCOPHEROL (UNII: R0ZB2556P8) POLYGLYCERYL-4 CAPRATE (UNII: 3N873UN885) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYDEXTROSE (UNII: VH2XOU12IE) PHENOXYETHANOL (UNII: HIE492ZZ3T) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) COUMARIN (UNII: A4VZ22K1WT) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83010-010-00 1 in 1 CARTON 01/01/2023 1 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 01/01/2023 Labeler - Maelys Cosmetics, LTD (532018258)