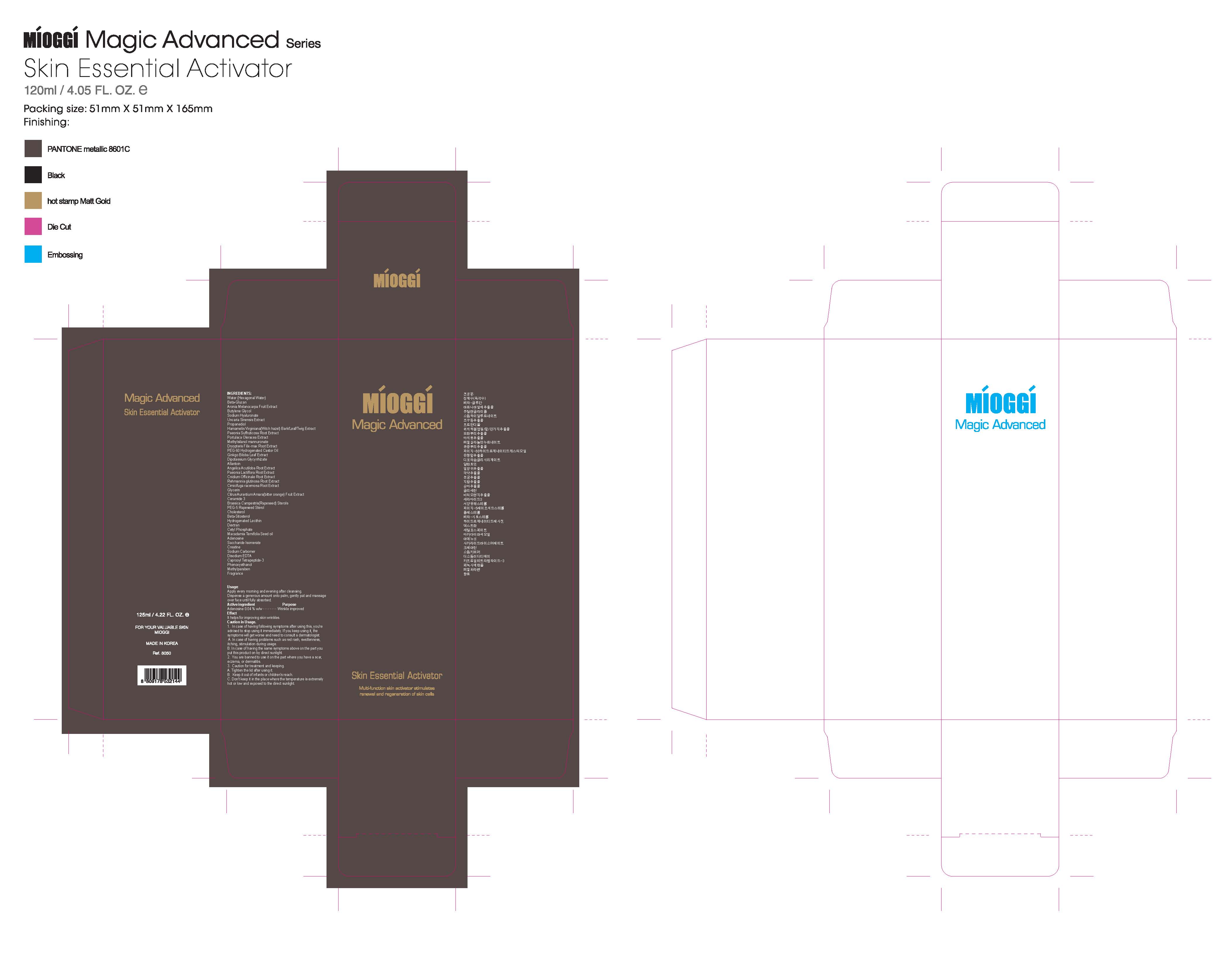
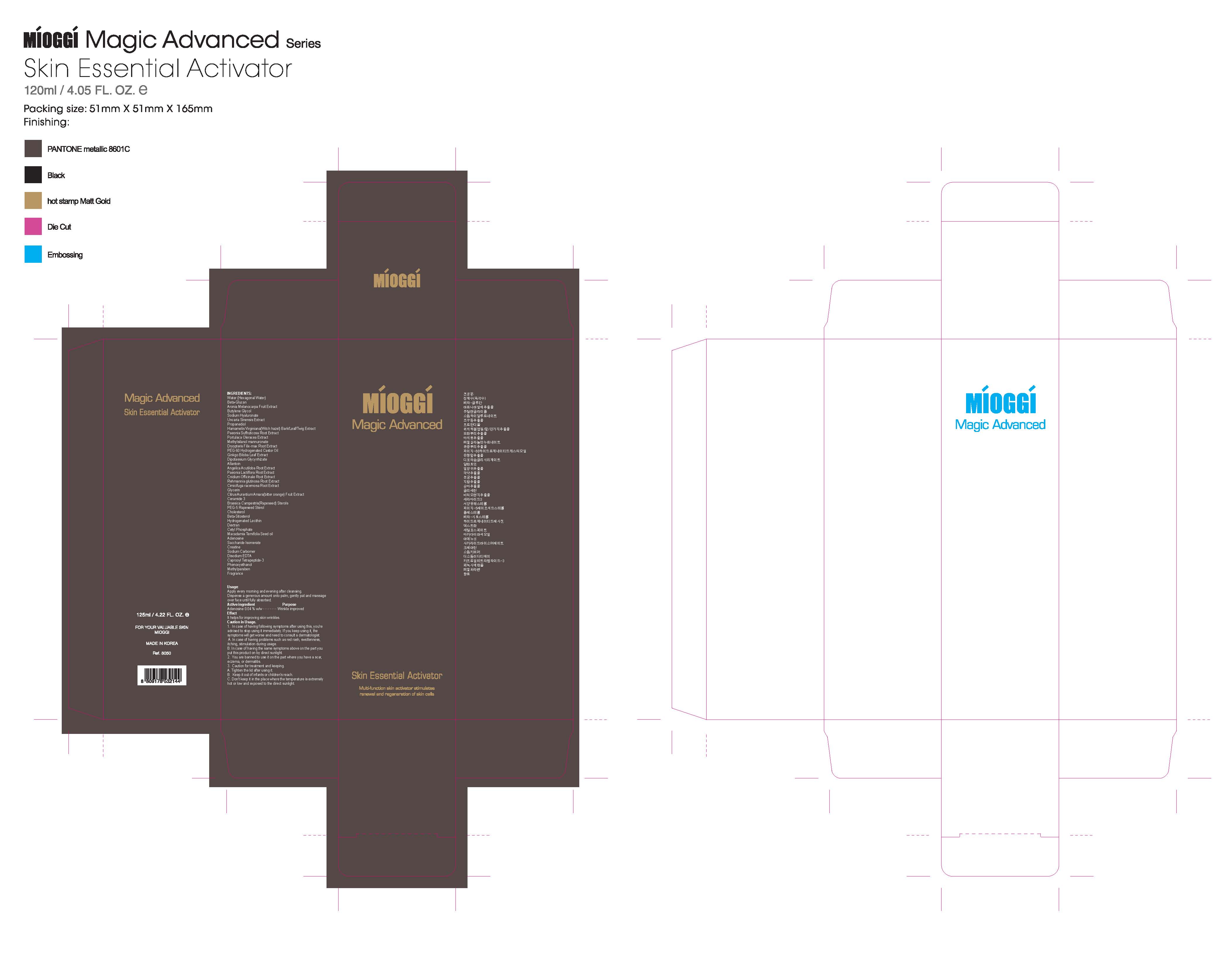
Label: MIOGGI MAGIC ADVANCED SKIN ESSENTIAL ACTIVATOR- adenosine liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 53009-2001-1 - Packager: Colorpink R&D Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 20, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Water, Methylsilanol mannuronate, Beta-glucan, Butylene glycol, Propanediol, PEG-60 hydrogenated castor oil, Morus alba root extract, Diospyros kaki leaf extract, Camellia sinensis leaf extract, Aloe barbadensis leaf extract, Dipotassium glycyrrhizate, Allantoin, Sodium hyaluronate, Adenosine, Sodium carbomer, Uncaria sinensis extract, Portulaca oleracea extract, Paeonia lactiflora root extract, Hamamelis virginiana(Witch hazel) bark/leaf/twig extract, Ginkgo biloba leaf extract, Citrus aurantium amara (Bitter orange) fruit extract, Rehmannia glutinosa root extract, Paeonia suffruticosa bark extract, Dryopteris crassirhizoma extract, Dryopteris filix-mas root extract, Cnidium officinale root extract, Cimicifuga racemosa root extract, Angelica acutiloba root extract, Macadamia ternifolia seed oil, Caprylic/capric triglyceride, Glycerin, PEG-5 rapeseed sterol, Cholesterol, Brassica campestris (Rapeseed) sterols, Beta-sitosterol, Saccharide isomerate, Hydrogenated lecithin, Ceramide 3, Choleth-24, Ceteth-24, Aronia melanocarpa fruit extract, Dextran, Polyglutamic acid, Creatine, Cetyl phosphate, Caprooyl tetrapeptide-3, Carthamus tinctorius (Safflower) seed extract, Disodium EDTA, Phenoxyethanol, Methylparaben, Proplyparaben, BHT, Fragrance
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
A. In case of having problems such as red rash, swollenness, itching, stimulation during usage.
B. In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. Caution for treatment and keeping.
A. Tighten the lid after using it.
B. Keep it out of infants or children's reach.
C. Don't keep it in the place where the temperature is extremely hot or low and exposed to the direct sunlight.
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MIOGGI MAGIC ADVANCED SKIN ESSENTIAL ACTIVATOR
adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:53009-2001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.05 mg in 125 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PROPANEDIOL (UNII: 5965N8W85T) POLYOXYL 60 CASTOR OIL (UNII: VXP26NM2XX) MORUS ALBA ROOT (UNII: CST1G9BZGD) DIOSPYROS KAKI LEAF (UNII: Q71GF9OBNO) GREEN TEA LEAF (UNII: W2ZU1RY8B0) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) ALLANTOIN (UNII: 344S277G0Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) UNCARIA SINENSIS WHOLE (UNII: 69OQ216G2K) PORTULACA OLERACEA WHOLE (UNII: D5J3623SV2) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) WITCH HAZEL (UNII: 101I4J0U34) GINKGO (UNII: 19FUJ2C58T) BITTER ORANGE OIL (UNII: 9TLV70SV6I) REHMANNIA GLUTINOSA ROOT (UNII: 1BEM3U6LQQ) PAEONIA SUFFRUTICOSA ROOT BARK (UNII: BUG255FE7X) DRYOPTERIS CRASSIRHIZOMA WHOLE (UNII: V3B80C759E) DRYOPTERIS FILIX-MAS ROOT (UNII: C0ZK0RRF5X) CNIDIUM OFFICINALE ROOT (UNII: 8S3OZD358J) BLACK COHOSH (UNII: K73E24S6X9) ANGELICA ACUTILOBA ROOT (UNII: 3W51R3EK30) MACADAMIA NUT (UNII: Y5432RGW8N) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) GLYCERIN (UNII: PDC6A3C0OX) CHOLESTEROL (UNII: 97C5T2UQ7J) RAPESEED STEROL (UNII: B46B6DD20U) .BETA.-SITOSTEROL (UNII: S347WMO6M4) SACCHARIDE ISOMERATE (UNII: W8K377W98I) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CERAMIDE 3 (UNII: 4370DF050B) CHOLETH-24 (UNII: 5UE7I54O43) CETETH-24 (UNII: 0EV3Z43Y2I) ARONIA MELANOCARPA FRUIT (UNII: S935718Z2Q) DEXTRAN 40 (UNII: K3R6ZDH4DU) CREATINE (UNII: MU72812GK0) CETYL PHOSPHATE (UNII: VT07D6X67O) CAPROOYL TETRAPEPTIDE-3 (UNII: LZI0HJ3K2R) CARTHAMUS TINCTORIUS SEEDCAKE (UNII: DHQ13F4N2W) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53009-2001-1 125 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/20/2012 Labeler - Colorpink R&D Inc. (557819375) Registrant - Colorpink R&D Inc. (557819375) Establishment Name Address ID/FEI Business Operations Colorpink R&D Inc. 557819375 manufacture(53009-2001)