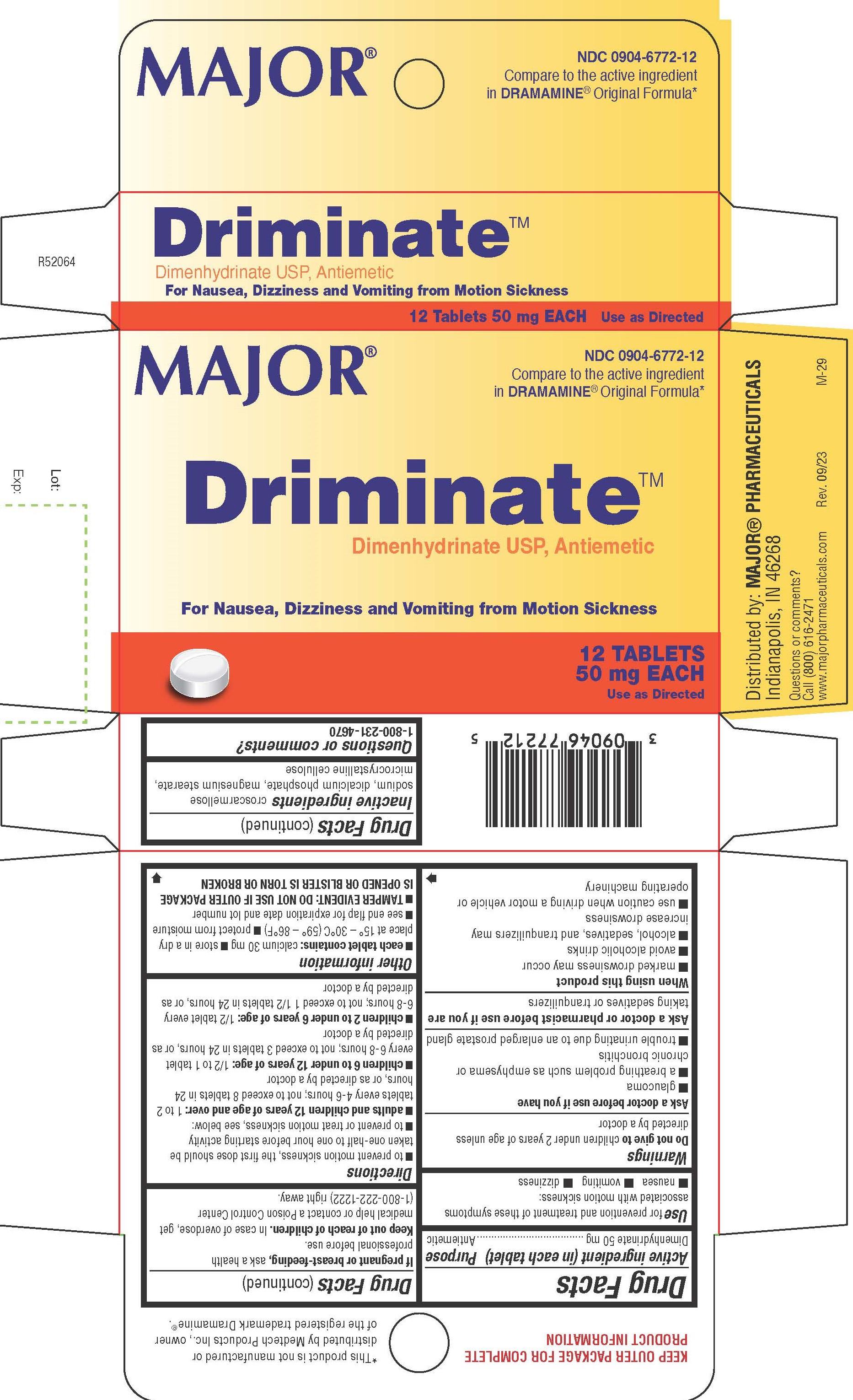
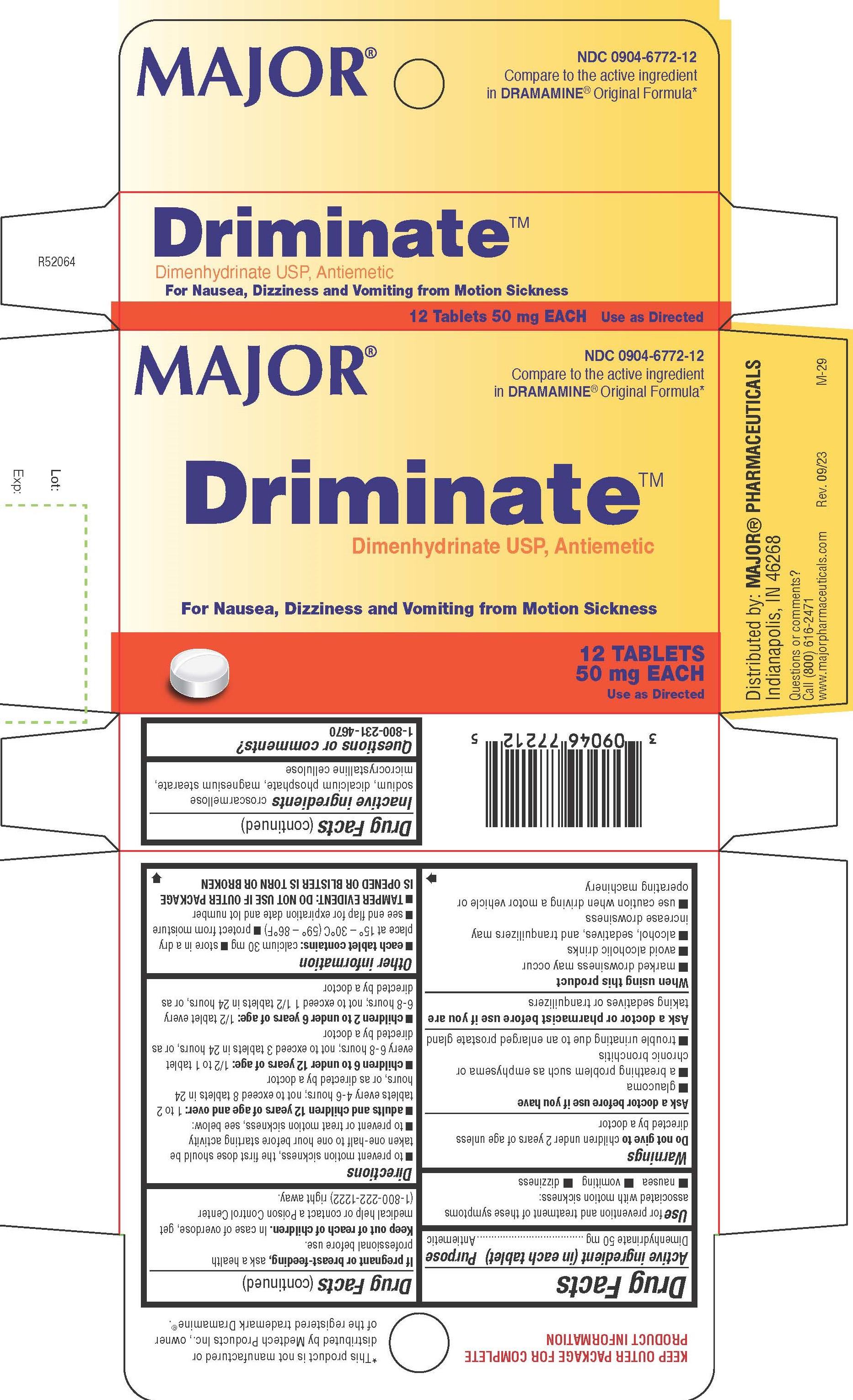
Label: DIMENHYDRINATE tablet
- NDC Code(s): 0904-6772-12
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
-
WARNINGS
Do not give to children under 2 years of age unless directed by a doctor
Ask a doctor before use if you have
■ glaucoma
■ a breathing problem such as emphysema or chronic bronchitis
■ trouble urinating due to an enlarged prostate gland
-
Directions
■ to prevent motion sickness, the rst dose should be taken one-half to one hour before starting activity
■ adults and children 12 years of age and over: 1 to 2 tablets every 4-6 hours; not to exceed 8 tablets in 24 hours, or as directed by a doctor
■ children 6 to under 12 years of age: 1/2 to 1 tablet every 6-8 hours; not to exceed 3 tablets in 24 hours, or as directed by a doctor
■ children 2 to under 6 years of age: 1/2 tablet every 6-8 hours; not to exceed 1 1/2 tablets in 24 hours, or as directed by a doctor
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
KEEP OUTER PACKAGE FOR COMPLETE PRODUCT INFORMATION
*This product is not manufactured or distributed by Medtech Products Inc., owner of the registered trademark Dramamine®.
Distributed by: MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268
Questions or comments?
Call (800) 616-2471
www.majorpharmaceuticals.com - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIMENHYDRINATE
dimenhydrinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-6772 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (CHLORTHEOPHYLLINE - UNII:GE2UA340FM) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color white Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code 1006;1006 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6772-12 1 in 1 CARTON 02/14/2019 1 12 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 02/14/2019 Labeler - Major Pharmaceuticals (191427277)