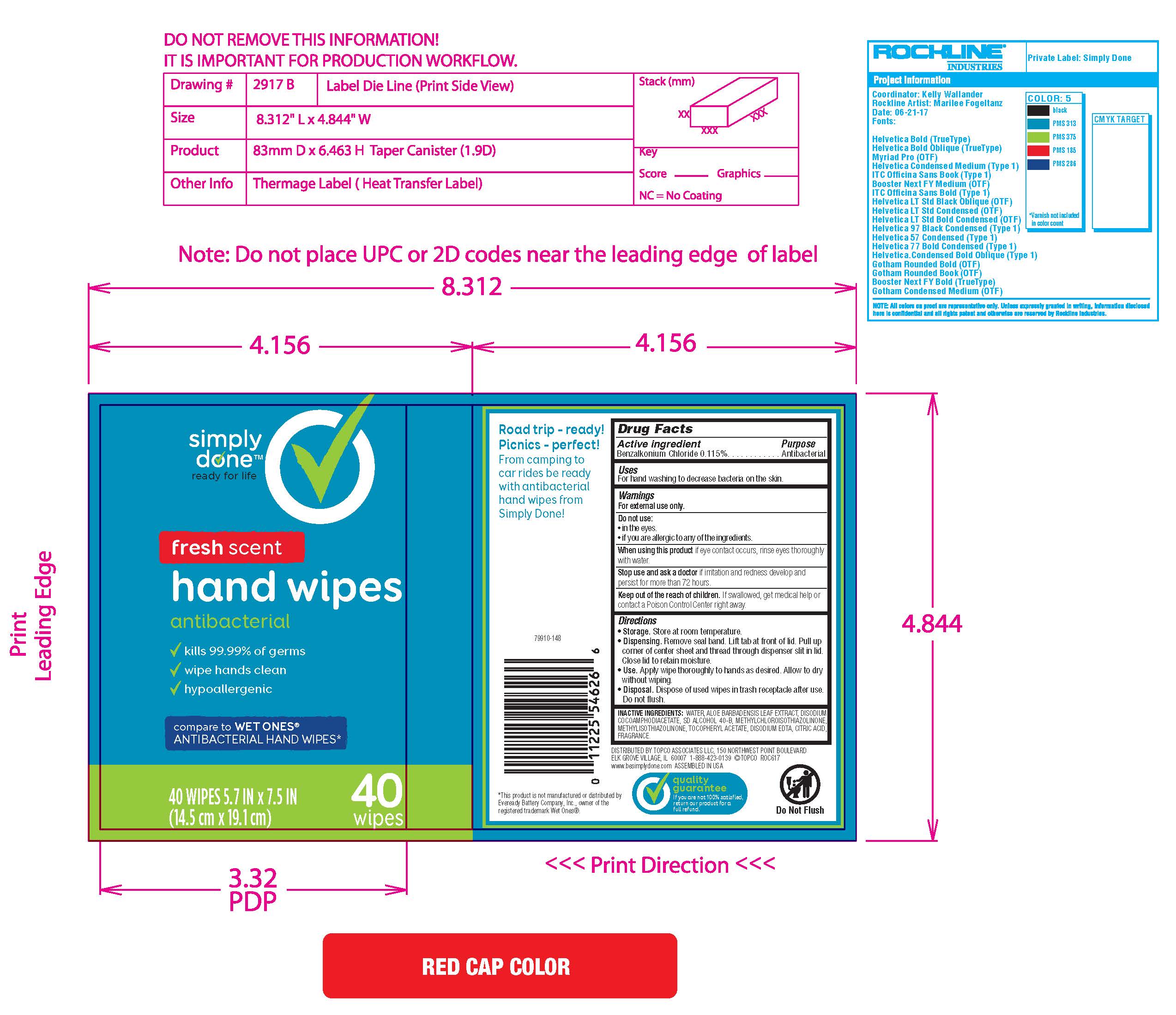
Label: SIMPLY DONE- benzalkonium chloride cloth
-
Contains inactivated NDC Code(s)
NDC Code(s): 36800-053-40 - Packager: Topco Associates, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 18, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
- Storage, Store at room temperature
- Dispensing Remove seal band. Lift tab at front of lid. Pull up corner of center sheet and thread through dispenser slit in lid. Close lid to retain moisture.
- Use. Apply wipe thoroughly to hands as desired. Allow to dry without wiping.
- Disposal. Dispose of used wipes in a trash receptacle after use. Do not flush.
- Inactive ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
SIMPLY DONE
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36800-053 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.115 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM (UNII: 7FLD91C86K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36800-053-40 40 in 1 CANISTER; Type 0: Not a Combination Product 05/27/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 05/27/2013 Labeler - Topco Associates, LLC (006935977)