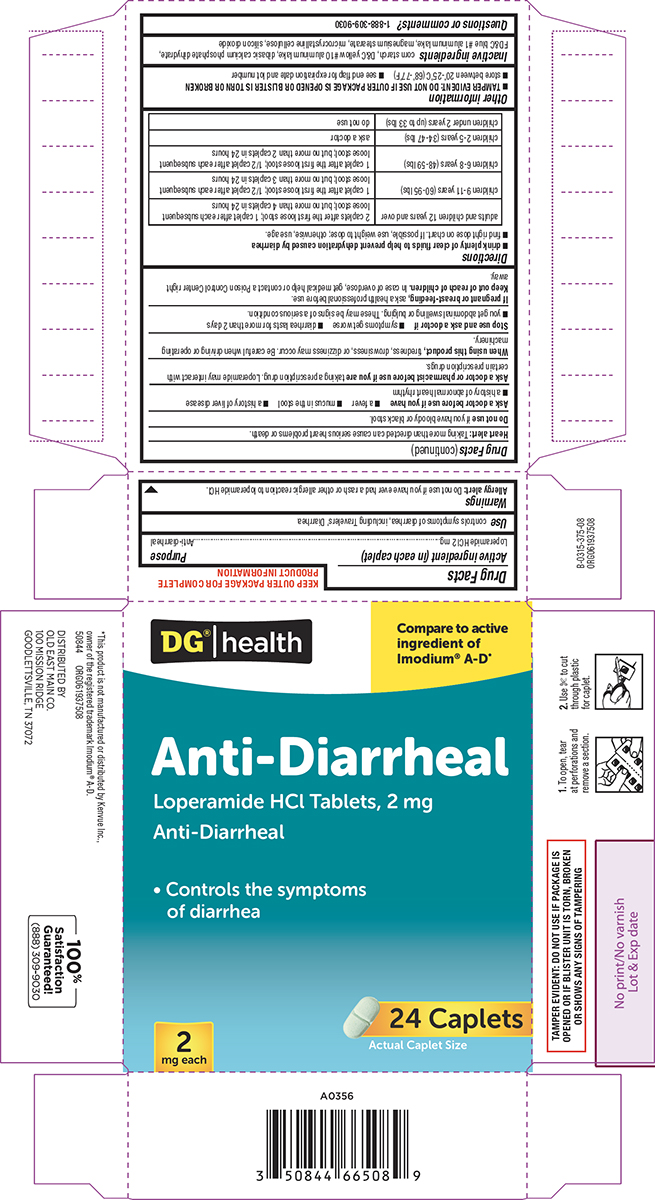
Label: ANTI-DIARRHEAL- loperamide hcl tablet
- NDC Code(s): 55910-375-02, 55910-375-08
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.Ask a doctor before use if you have
- a fever
- mucus in the stool
- a history of liver disease
- a history of abnormal heart rhythm
Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool; 1 caplet after each subsequent loose stool; but no more than 4 caplets in 24 hours children 9-11 years (60-95 lbs) 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 3 caplets in 24 hours children 6-8 years (48-59 lbs) 1 caplet after the first loose stool; 1/2 caplet after each subsequent loose stool; but no more than 2 caplets in 24 hours children 2-5 years (34-47 lbs) ask a doctor children under 2 years (up to 33 lbs) do not use - Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
DG® | health
Compare to active
ingredient of
Imodium® A-D*Anti-Diarrheal
Loperamide HCl Tablets, 2 mg
Anti-Diarrheal• Controls the symptoms
of diarrhea2
mg each24 Caplets
Actual Caplet SizeTAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING1. To open, tear
at perforations and
remove a section.2. Use ✄ to cut
through plastic
for caplet.*This product is not manufactured or distributed by Kenvue Inc.,
owner of the registered trademark Imodium® A-D.
50844 ORG061937508DISTRIBUTED BY
OLD EAST MAIN CO.
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072100% Satisfaction
Guaranteed!
(888)309-9030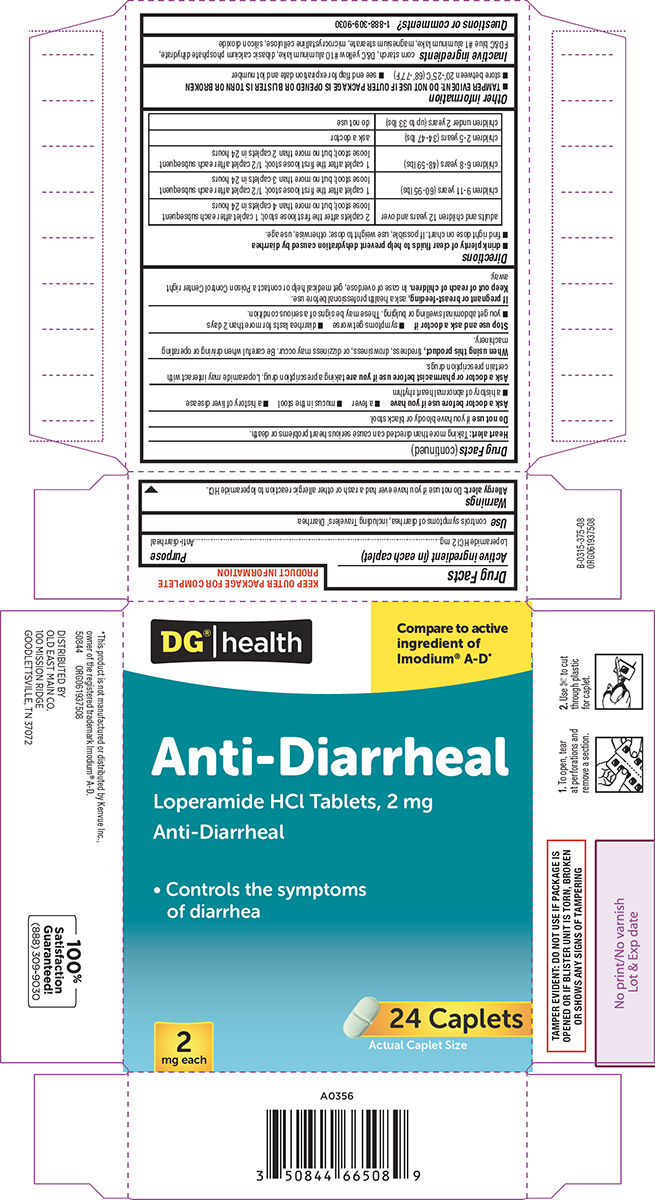
Dollar General 44-375
-
INGREDIENTS AND APPEARANCE
ANTI-DIARRHEAL
loperamide hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-375 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color green Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 44;375 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-375-02 2 in 1 CARTON 01/12/2024 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:55910-375-08 4 in 1 CARTON 01/12/2024 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076497 01/12/2024 Labeler - DOLGENCORP, LLC (068331990) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(55910-375) , pack(55910-375) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(55910-375)