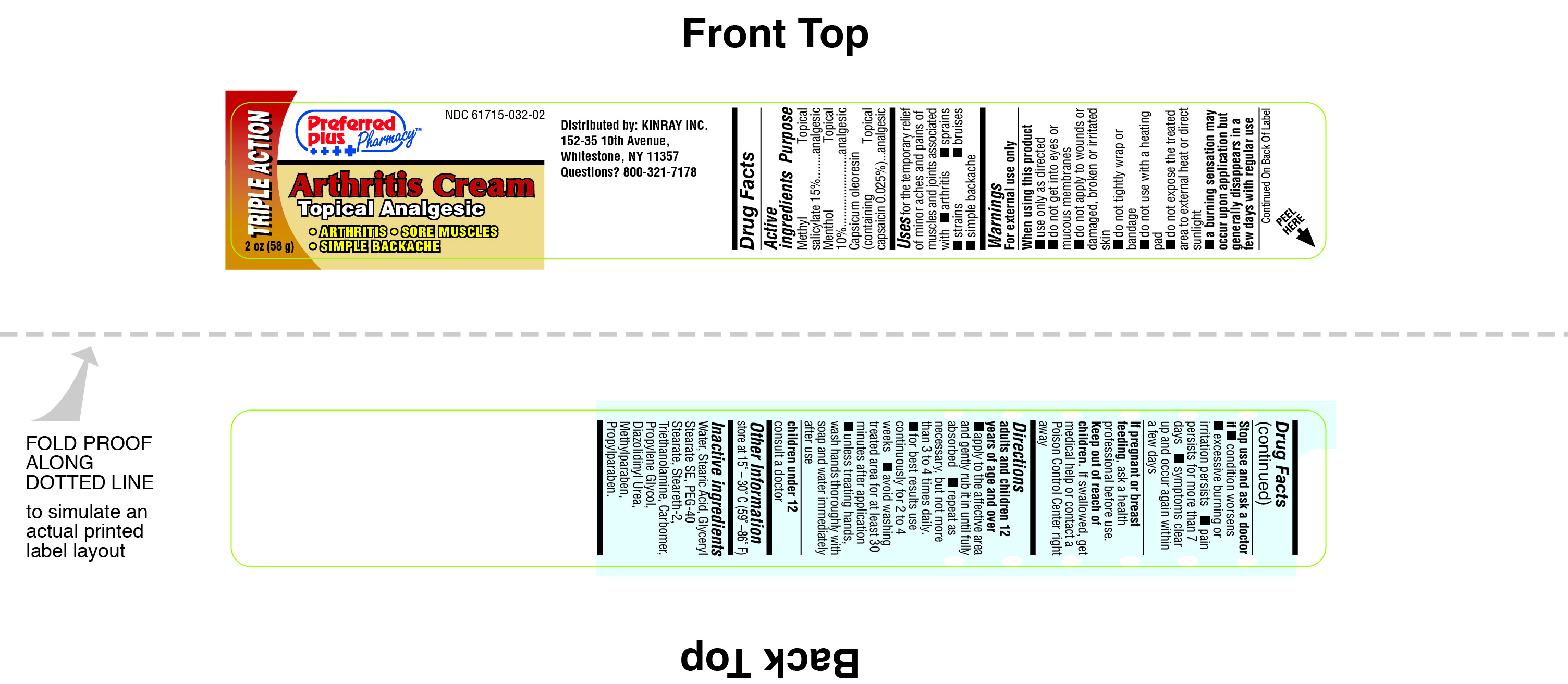
Label: PREFERRED PLUS ARTHRITIS CREAM- menthol topical analgesic cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 61715-032-02 - Packager: Kinray
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 1, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warning
- Ask Doctor
- If pregnant or breast feeding
- Keep out of reach of children
-
Directions
- apply to the affective area and gently rub it in until fully absorbed
- repeat asnecessary, but not more than 3 to 4 times daily.
- for best results use continuously for 2 to 4 weeks
- avoid washing treated area for at least 30 minutes after application
- unless treating hands, wash hands thoroughly with soap and water immediately after use
Children under 12
consult a doctor - Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREFERRED PLUS ARTHRITIS CREAM
menthol topical analgesic creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61715-032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 15 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) PEG-40 STEARATE (UNII: ECU18C66Q7) STEARETH-2 (UNII: V56DFE46J5) TROLAMINE (UNII: 9O3K93S3TK) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) CARBOMER 940 (UNII: 4Q93RCW27E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61715-032-02 58 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/23/2001 Labeler - Kinray (012574513) Registrant - Reese Pharmaceutical Co (004172052) Establishment Name Address ID/FEI Business Operations Reese Pharmaceutical Co 004172052 relabel(61715-032) , repack(61715-032)