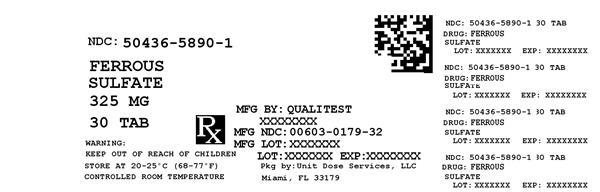
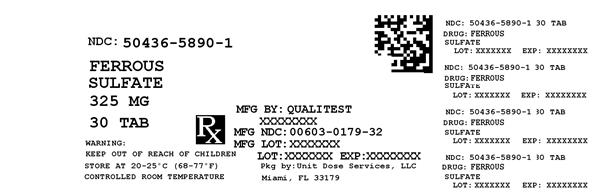
Label: FERROUS SULFATE- iron supplement tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 50436-5890-1 - Packager: Unit Dose Services
- This is a repackaged label.
- Source NDC Code(s): 0603-0179
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 12, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Supplement Facts[/S]
- SUGGESTED USE
- Active Ingredient
- Inactive Ingredients
- Purpose
-
WARNING:
Accidental overdose of iron containing products is a leading cause of fatal poisoning in children under 6. . In case of accidental overdose, call a doctor or Poison Control Center immediately. Keep this product out of reach of children
The treatment of any anemic condition should be under the advice and supervision of doctor. Occasional gastrointestinal discomfort (such as nausea) may be minimized by taking with meals. Iron-containing medication may occasionally cause constipation or diarrhea. WARNINGS: Do not exceed recommended dosage.
As with any drug, if you are pregnant or nursing baby, seek the advice of a health professional before using this product.
- DRUG INTERACTION PRECAUTION
- DOSAGE AND ADMINISTRATION
- Questions or Comments
- FERROUS SULFATE (IRON SUPPLEMENT) TABLET
-
INGREDIENTS AND APPEARANCE
FERROUS SULFATE
iron supplement tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50436-5890(NDC:0603-0179) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS SULFATE (UNII: 39R4TAN1VT) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 325 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color RED Score no score Shape ROUND Size 8mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50436-5890-1 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 02/19/2001 Labeler - Unit Dose Services (831995316) Registrant - Unit Dose Services (831995316) Establishment Name Address ID/FEI Business Operations Unit Dose Services 831995316 REPACK(50436-5890)