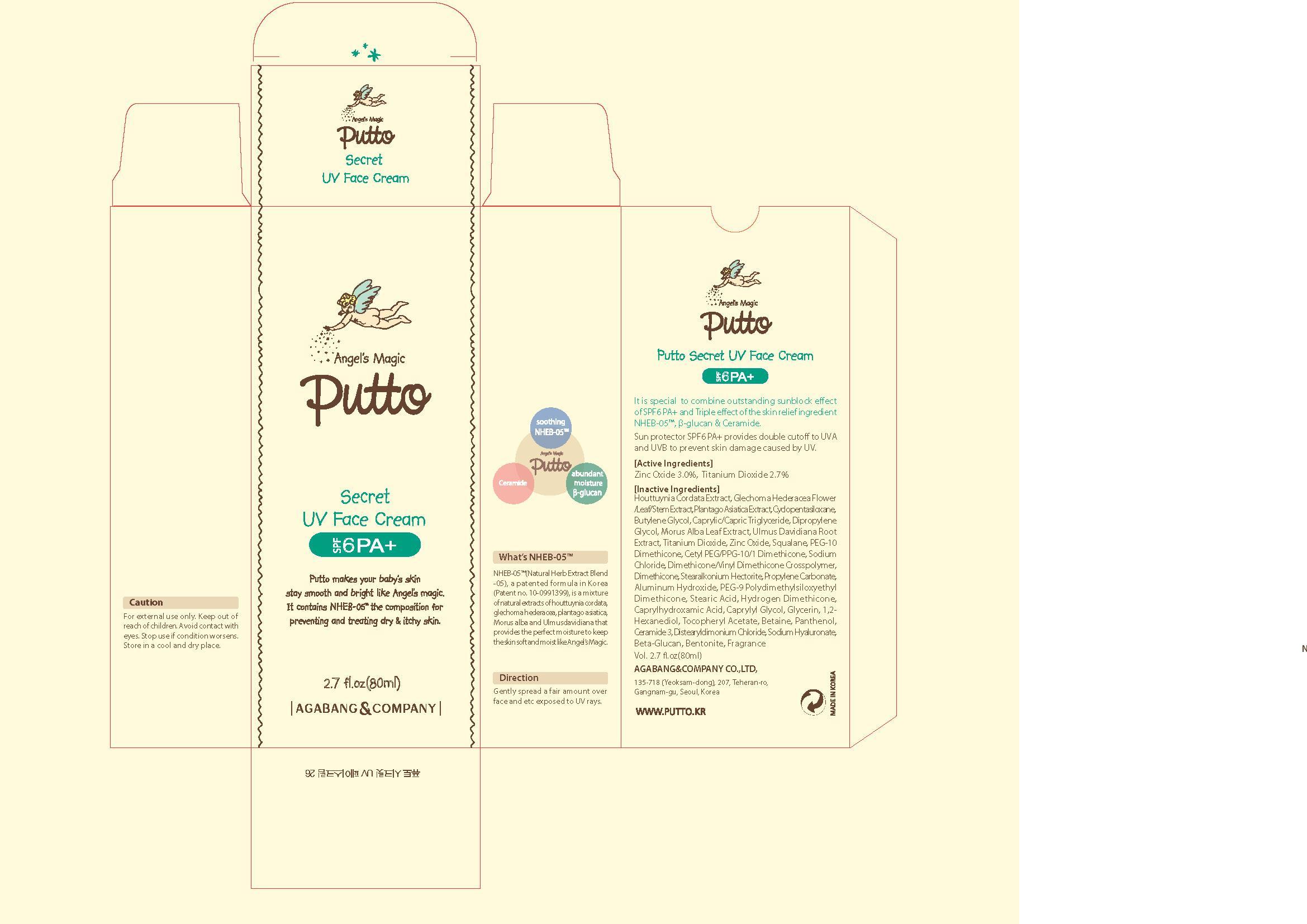
Label: PUTTO SECRET UV FACE- titanium dioxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 75885-040-01 - Packager: AGABANG & COMPANY
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 3, 2013
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
houttuynia cordata ext, glechoma hederacea flower/leaf/stem ext, plantago asiatica ext, cyclopentasiloxane, butylene glycol, crprylic/capric triglyceride, dipropylene glycol, morus alba leaf ext, ulmus davidiana root ext, zinc oxide, squalane, peg-10 dimethicone, cetyl peg/ppg-10/1 dimethicone, sodium chloride, dimethicone/vinyl dimethicone crosspolymer, dimethicone, stearalkonium hectorite, propylene carbonate, aluminum hydroxide, peg-9 polydimethylsiloxyethyl dimethicone, stearic acid,hydrogen dimethicone, caprylhydroxamic acid, caprylyl glycol, glycerin, 1,2-hexanediol, tocopheryl acetate betaine, panthenol, ceramide 3, distearyldimonium chloride, sodium hyaluronate, beta-glucan, bentonite, fragrance
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PUTTO SECRET UV FACE
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75885-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.96 mg in 100 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLECHOMA HEDERACEA FLOWERING TOP (UNII: 2458J91U39) HOUTTUYNIA CORDATA FLOWERING TOP (UNII: RH041UUZ22) PLANTAGO ASIATICA (UNII: 5F5728J5E8) SQUALENE (UNII: 7QWM220FJH) SODIUM CHLORIDE (UNII: 451W47IQ8X) DIMETHICONE (UNII: 92RU3N3Y1O) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLYCERIN (UNII: PDC6A3C0OX) MORUS ALBA LEAF (UNII: M8YIA49Q2P) ULMUS DAVIDIANA ROOT (UNII: URQ79U8261) ALGELDRATE (UNII: 03J11K103C) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75885-040-01 80 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 09/03/2013 Labeler - AGABANG & COMPANY (687808766) Registrant - AGABANG & COMPANY (687808766) Establishment Name Address ID/FEI Business Operations AGABANG & COMPANY 687808766 manufacture(75885-040)