Label: ANTI-BLEMISH CONCEALER- salicylic acid stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 69863-201-11, 69863-201-51 - Packager: HATCHBEAUTY PRODUCTS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 23, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USES
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
Directions
• Clean the skin thoroughly before applying this product
• Cover the entire affected area with a thin layer one to three times daily
• Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
• If bothersome dryness or peeling occurs, reduce application to once a day or every other day
• Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during first 3 days. If no discomfort occurs, follow directions above.
-
Inacitve Ingredients
Phenyl Trimethicone, Octyldodecanol, C12-15 Alkyl Benzoate, Stearyl Dimethicone, Euphorbia Cerifera (Candelilla) Wax, Ozokerite, Beeswax, Polyethylene, Allyl Methacrylates Crosspolymer, Tridecyl Stearate, Camellia Oleifera Leaf Extract, Ascorbyl Palmitate, Tocopherol, Tocopheryl Acetate, Retinyl Palmitate, Dimethicone, Dimethiconol, Caprylic/Capric Triglyceride, Cyclopentasiloxane, Propylene Carbonate, Disteardimonium Hectorite, Tridecyl Trimellitate, Neopentyl Glycol Dicaprylate/Dicaprate, Phenoxyethanol. (May Contain): Iron Oxides (CI 77491/77492/77499), Titanium Dioxide (CI 77891)
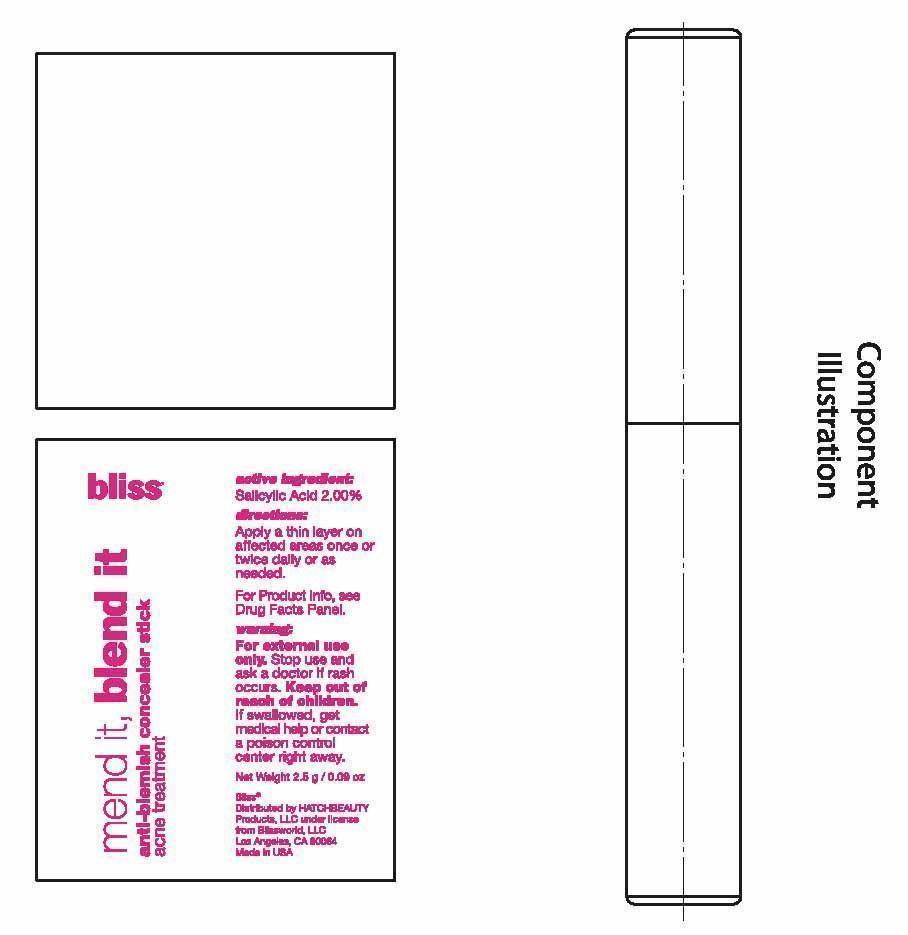
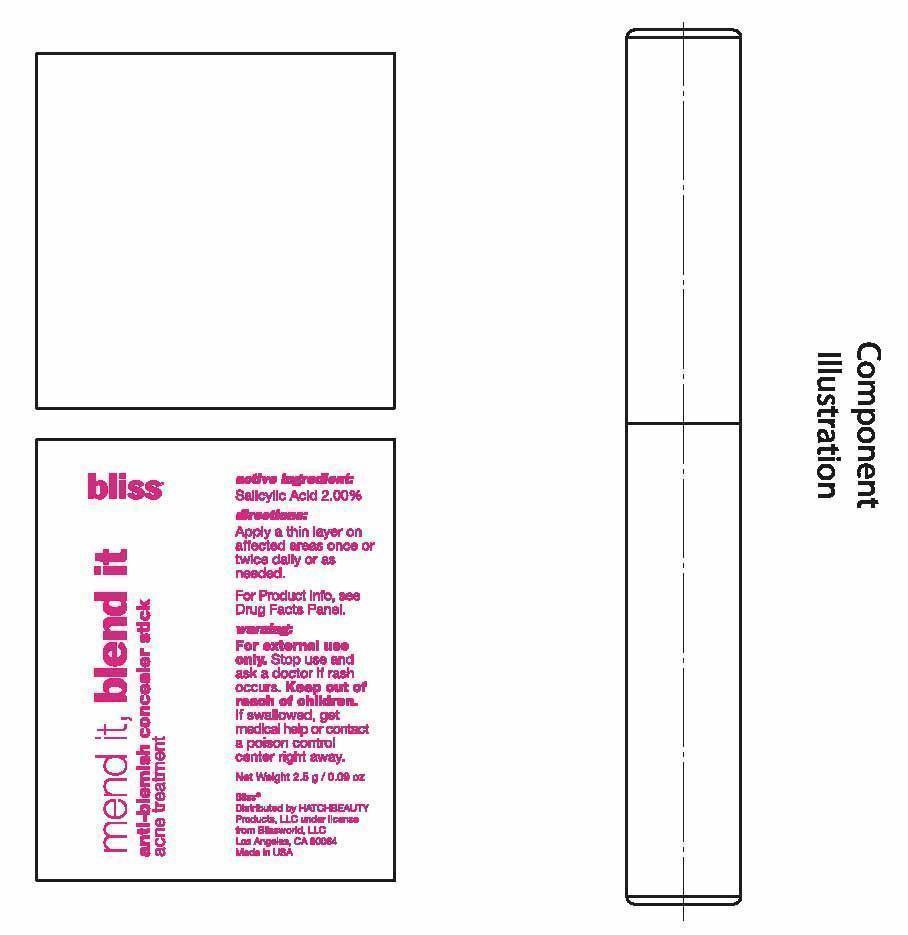
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ANTI-BLEMISH CONCEALER
salicylic acid stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69863-201 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) OCTYLDODECANOL (UNII: 461N1O614Y) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) STEARYL DIMETHICONE (400 MPA.S AT 50C) (UNII: R327X197HY) CANDELILLA WAX (UNII: WL0328HX19) CERESIN (UNII: Q1LS2UJO3A) YELLOW WAX (UNII: 2ZA36H0S2V) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) ALLYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: B9J55EA6QX) TRIDECYL STEARATE (UNII: A8OE252M6L) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHICONOL (2000 CST) (UNII: T74O12AN6Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) PROPYLENE CARBONATE (UNII: 8D08K3S51E) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69863-201-51 1 in 1 BOX 1 NDC:69863-201-11 2.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 06/18/2015 Labeler - HATCHBEAUTY PRODUCTS LLC (044612361) Registrant - HATCHBEAUTY PRODUCTS LLC (044612361)