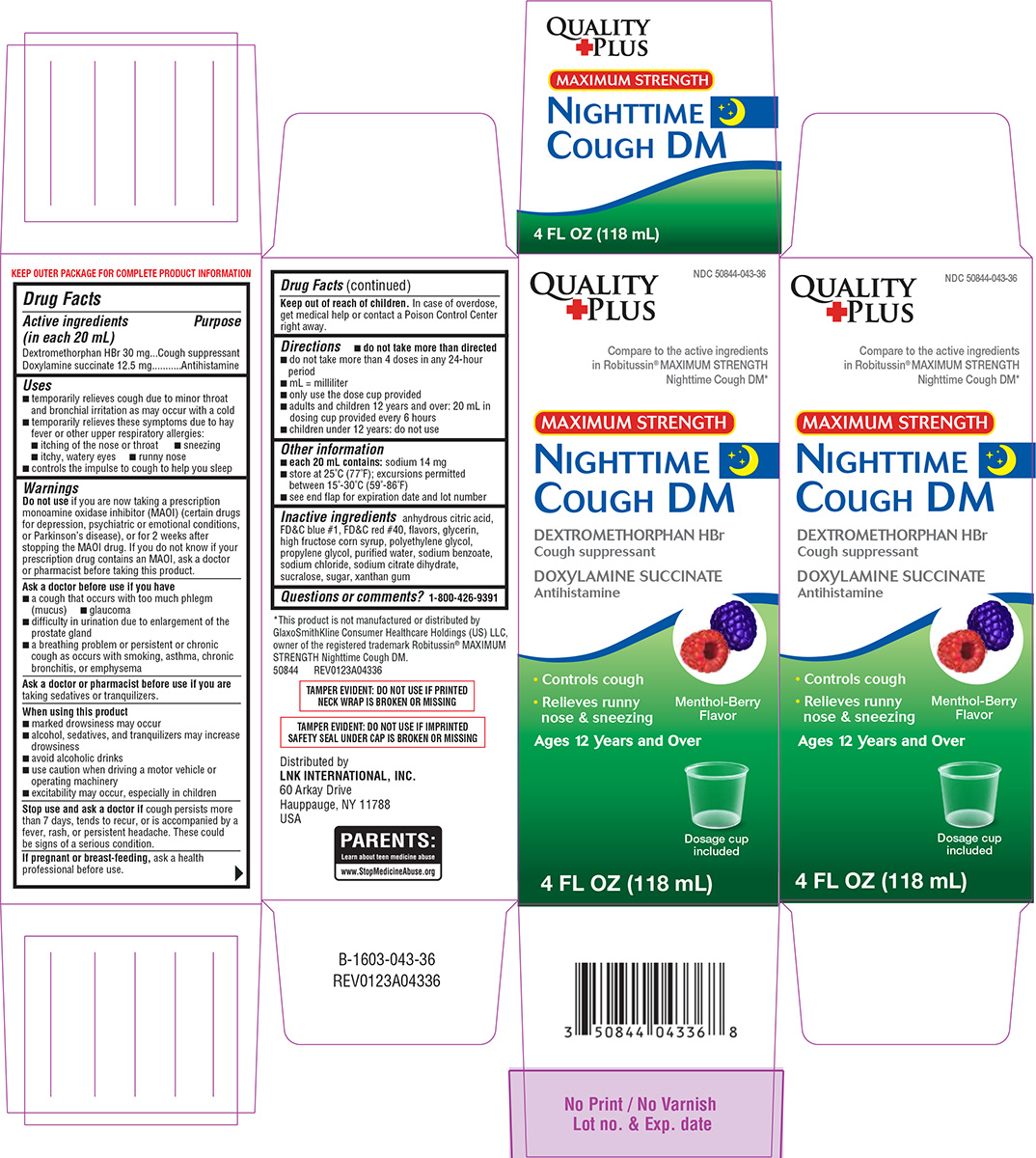
Label: NIGHTTIME COUGH DM- dextromethorphan hbr, doxylamine succinate solution
- NDC Code(s): 50844-043-36
- Packager: L.N.K. International, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 20 mL)
- Purpose
-
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- itching of the nose or throat
- sneezing
- itchy, watery eyes
- runny nose
- controls the impulse to cough to help you sleep
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that occurs with too much phlegm (mucus)
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a breathing problem or persistent or chronic cough as occurs with smoking, asthma, chronic bronchitis, or emphysema
When using this product
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use caution when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
NDC 50844-043-36
Quality
PlusCompare to the active ingredients
in Robitussin® MAXIMUM STRENGTH
Nighttime Cough DM*MAXIMUM STRENGTH
NIGHTTIME
COUGH DM
DEXTROMETHORPHAN HBr
Cough suppressant
DOXYLAMINE SUCCINATE
Antihistamine• Controls cough
• Relieves runny nose & sneezingMenthol-Berry
FlavorAges 12 Years and Over
4 FL OZ (118 mL)
Dosage cup
included*This product is not manufactured or
distributed by PF Consumer Healthcare 1 LLC,
owner of the registered trademark Robitussin®
MAXIMUM STRENGTH Nighttime Cough DM.
50844 REV0123A04336Distributed by
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USATAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSINGTAMPER EVIDENT: DO NOT USE IF PRINTED
NECK WRAP IS BROKEN OR MISSING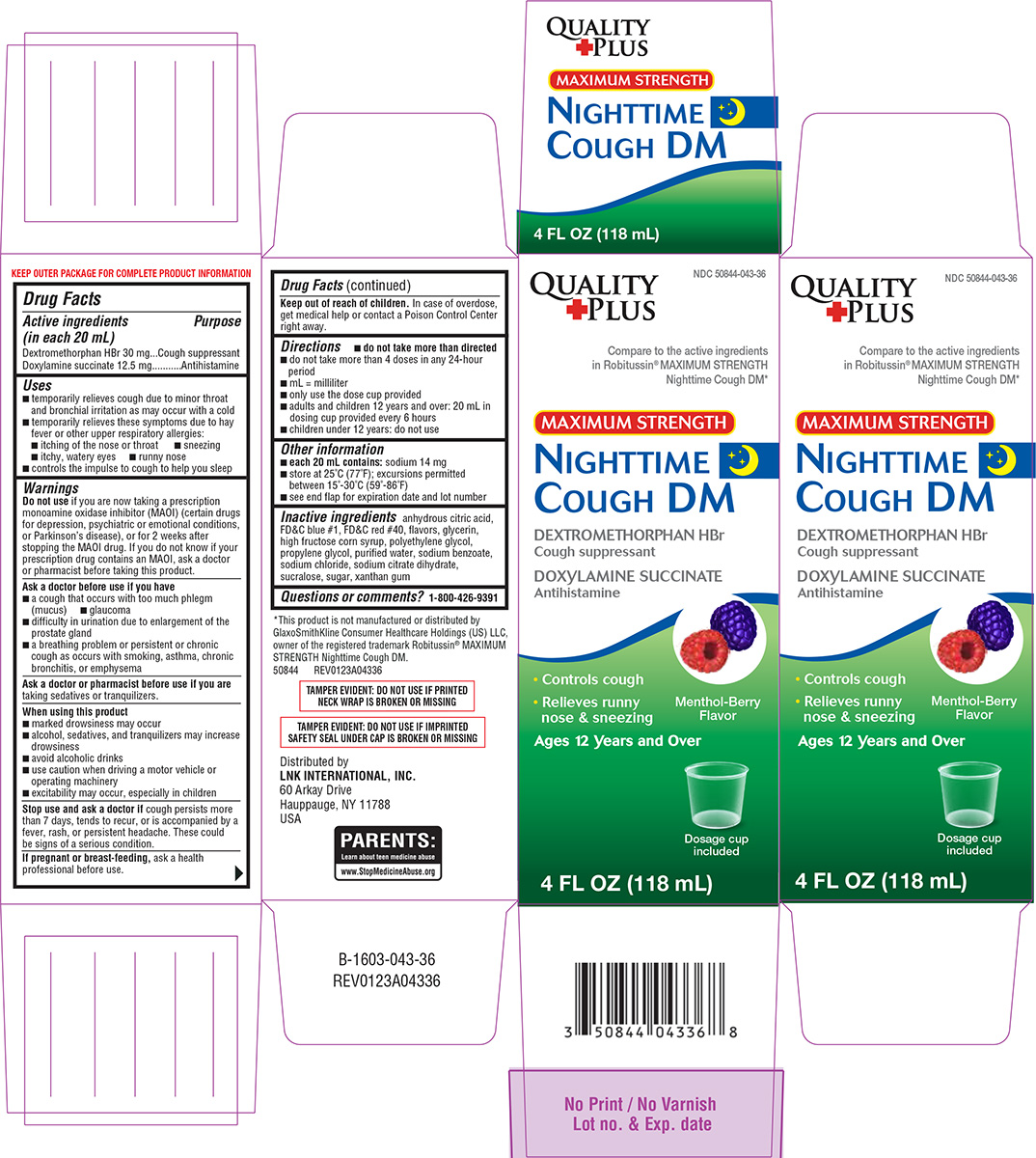
Quality Plus 44-043
-
INGREDIENTS AND APPEARANCE
NIGHTTIME COUGH DM
dextromethorphan hbr, doxylamine succinate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50844-043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 30 mg in 20 mL DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 12.5 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCRALOSE (UNII: 96K6UQ3ZD4) SUCROSE (UNII: C151H8M554) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color red (maroon) Score Shape Size Flavor BERRY, MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50844-043-36 1 in 1 CARTON 07/16/2021 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 07/16/2021 Labeler - L.N.K. International, Inc. (038154464) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 manufacture(50844-043) , pack(50844-043)