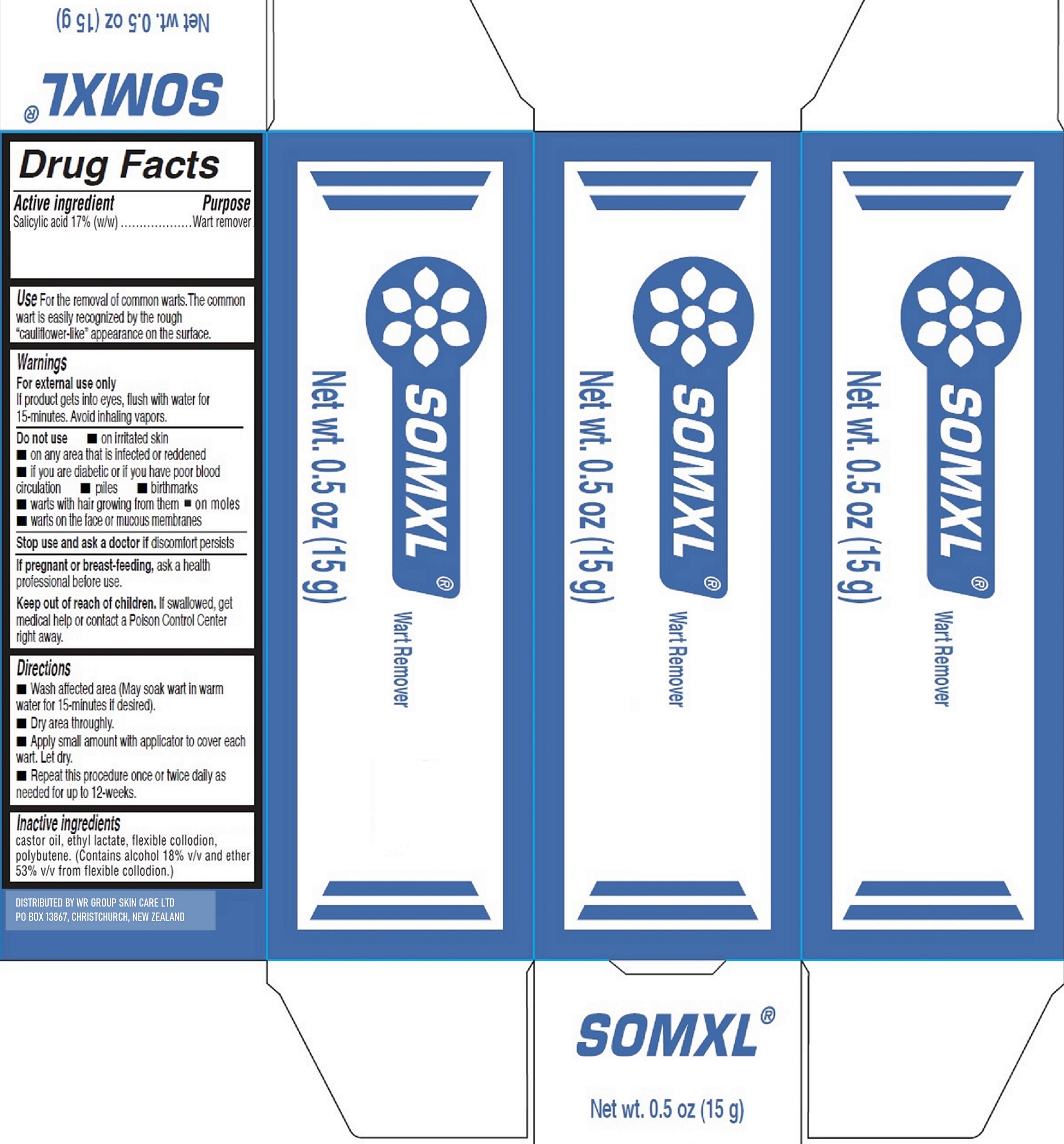
Label: SOMXL- salicylic acid solution
- NDC Code(s): 70463-000-01
- Packager: WR Group Skin Care Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
-
Warnings
For external use only
If product gets into eyes, flush with water for 15-minutes. Avoid inhaling vapors.
- Directions
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SOMXL
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70463-000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 170 mg in 1 g Inactive Ingredients Ingredient Name Strength CASTOR OIL (UNII: D5340Y2I9G) ETHYL LACTATE (UNII: F3P750VW8I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70463-000-01 1 in 1 BOX 06/26/2023 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 06/26/2023 Labeler - WR Group Skin Care Ltd (592719822)