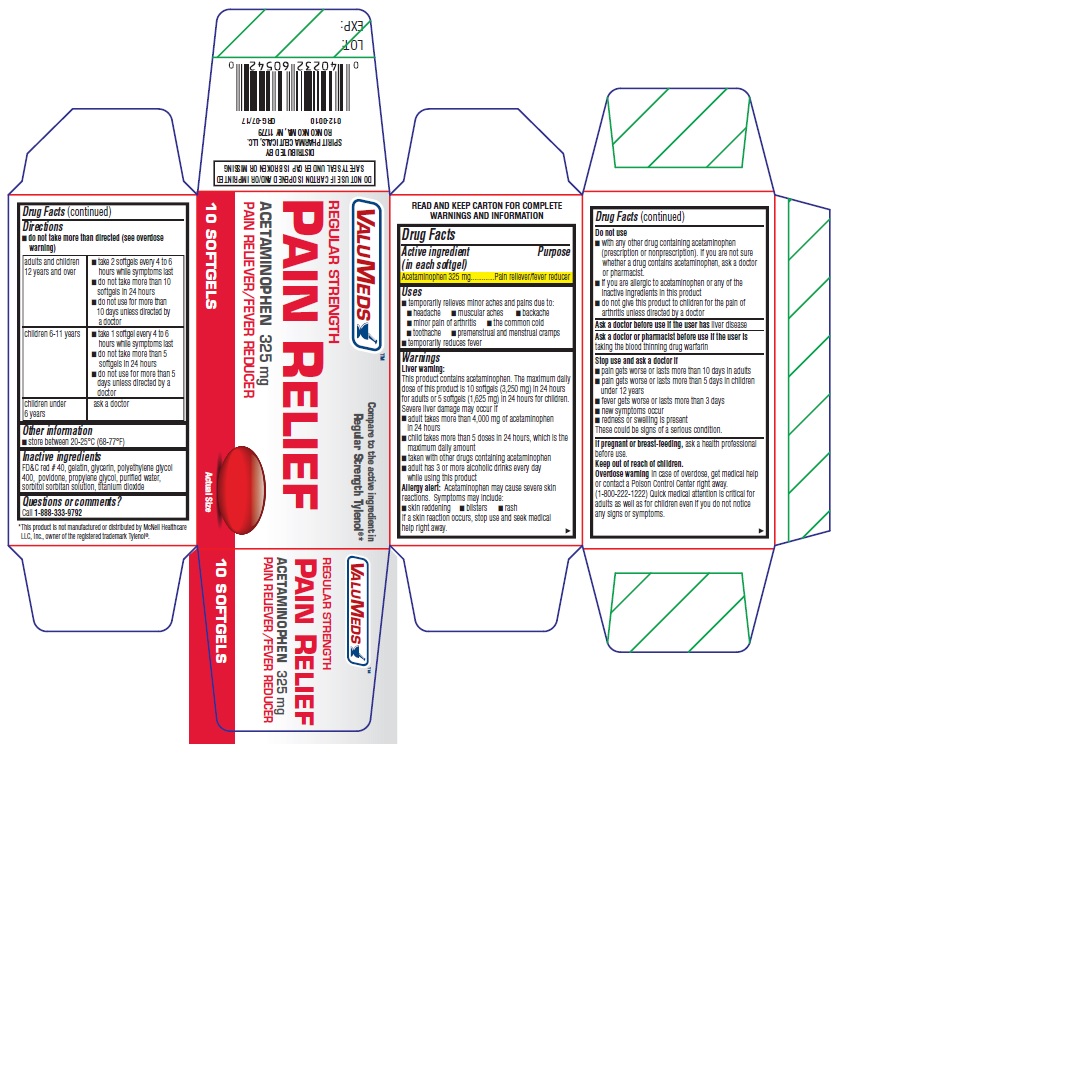
Label: REGULAR STRENGTH PAIN RELIEF- acetaminophen capsule, gelatin coated
- NDC Code(s): 68210-0120-1
- Packager: SPIRIT PHARMACEUTICALS LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each softgel)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. The maximum daily dose of this product is 10 softgels (3,250 mg) in 24 hours for adults or 5 softgels (1,625 mg) in 24 hours for children. Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
- do not give this product to children for the pain of arthritis unless directed by a doctor
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 softgels every 4 to 6 hours while symptoms last
- do not take more than 10 softgels in 24 hours
- do not use for more than 10 days unless directed by a doctor
children 6-11 years - take 1 softgel every 4 to 6 hours while symptoms last
- do not take more than 5 softgels in 24 hours
- do not use for more than 5 days unless directed by a doctor
children under 6 years ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 10 Softgel Bottle Carton
-
INGREDIENTS AND APPEARANCE
REGULAR STRENGTH PAIN RELIEF
acetaminophen capsule, gelatin coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-0120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE K30 (UNII: U725QWY32X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape CAPSULE (OBLONG) Size 21mm Flavor Imprint Code 800 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-0120-1 1 in 1 CARTON 04/10/2018 1 10 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 04/10/2018 Labeler - SPIRIT PHARMACEUTICALS LLC (179621011)