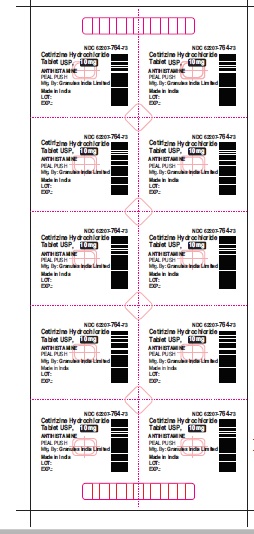
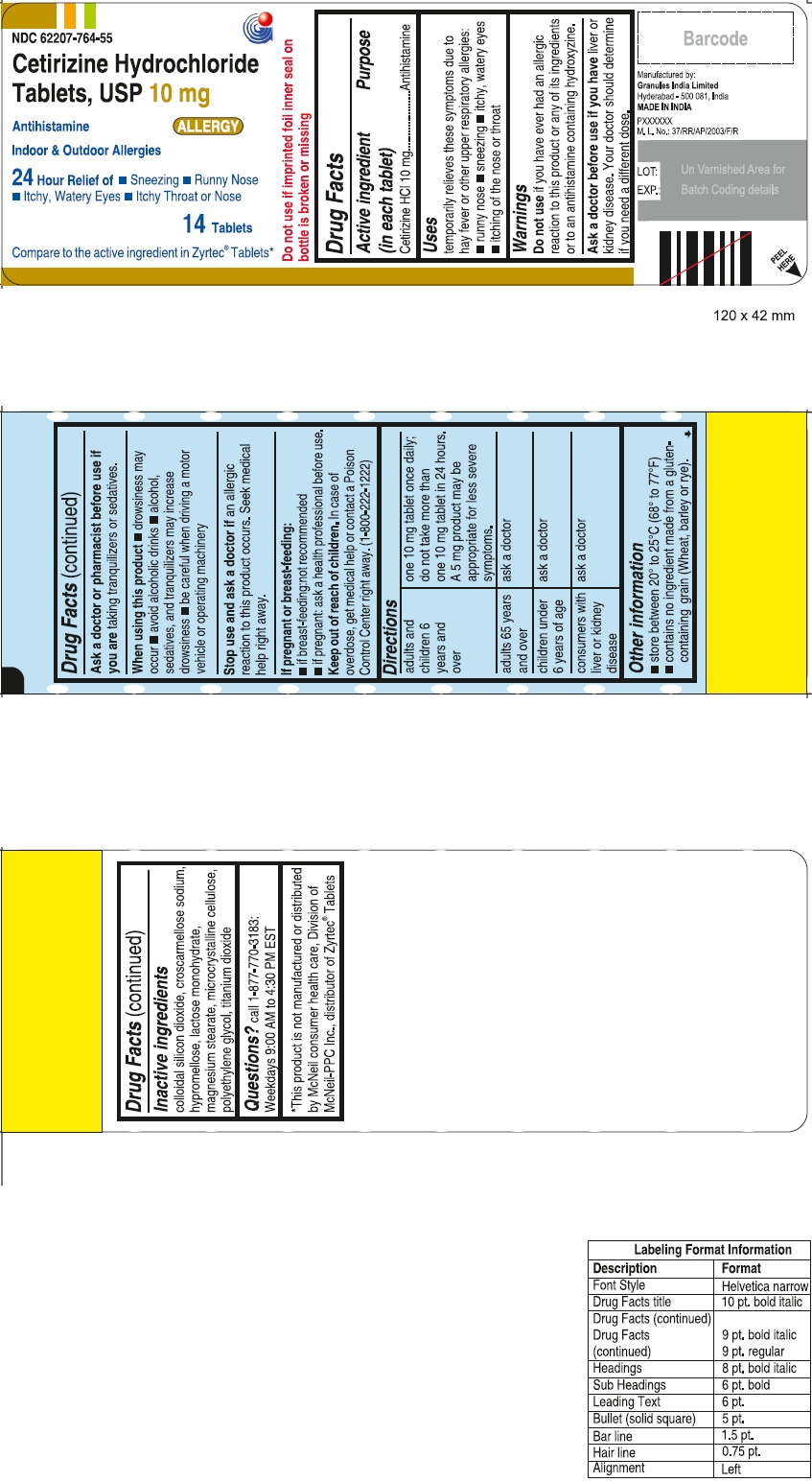
Label: CETIRIZINE HYDROCHLORIDE tablet, coated
- NDC Code(s): 62207-764-49, 62207-764-55
- Packager: Granules India Ltd
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 27, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
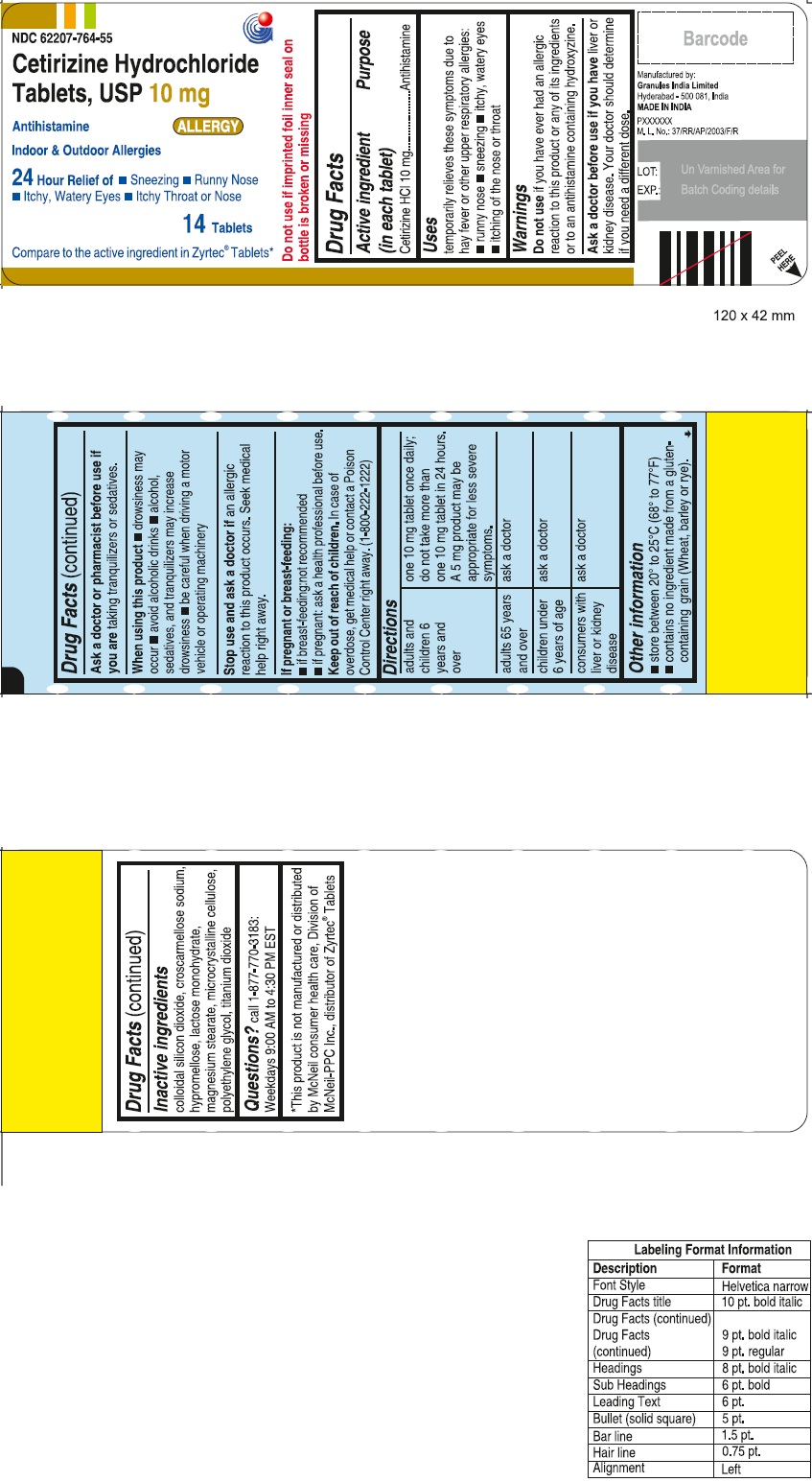
- Cetirizine Hydrochloride Tablets
- Active Ingredient
- PURPOSE
- USE(S)
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF
- ASK A DOCTOR OR PHARMACIST BEFORE USE IF
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DIRECTIONS
adults and children 6 years and over one 10 mg tablet once daily; do not take more than one 10 mg tablet in 24 hours. A 5 mg product may be appropriate for less severe symptoms. adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor
- STORAGE
- Other information
- Inactive ingredients
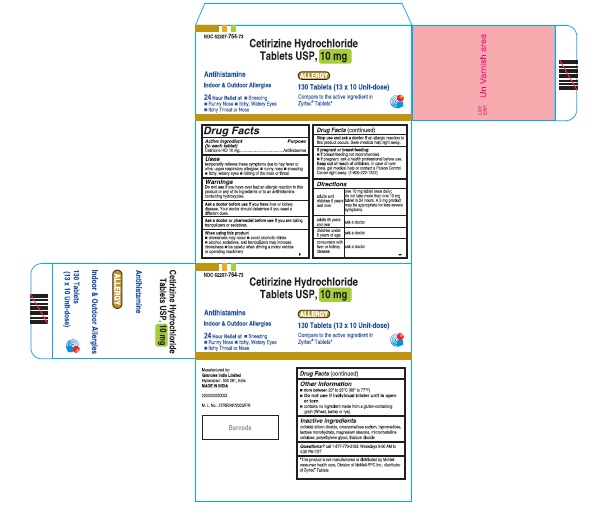
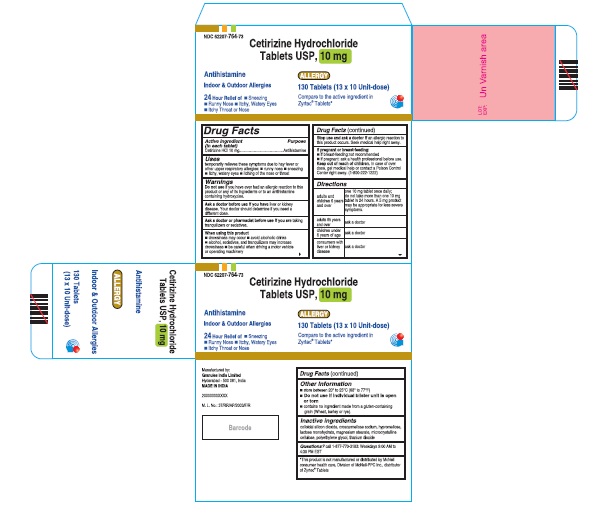
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62207-764 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) Product Characteristics Color white (white to off white) Score 2 pieces Shape RECTANGLE (rounded off rectangualr) Size 9mm Flavor Imprint Code G;4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62207-764-55 14 in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2018 2 NDC:62207-764-49 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/28/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209274 05/28/2018 Labeler - Granules India Ltd (915000087) Establishment Name Address ID/FEI Business Operations Granules India Ltd 918609236 manufacture(62207-764)