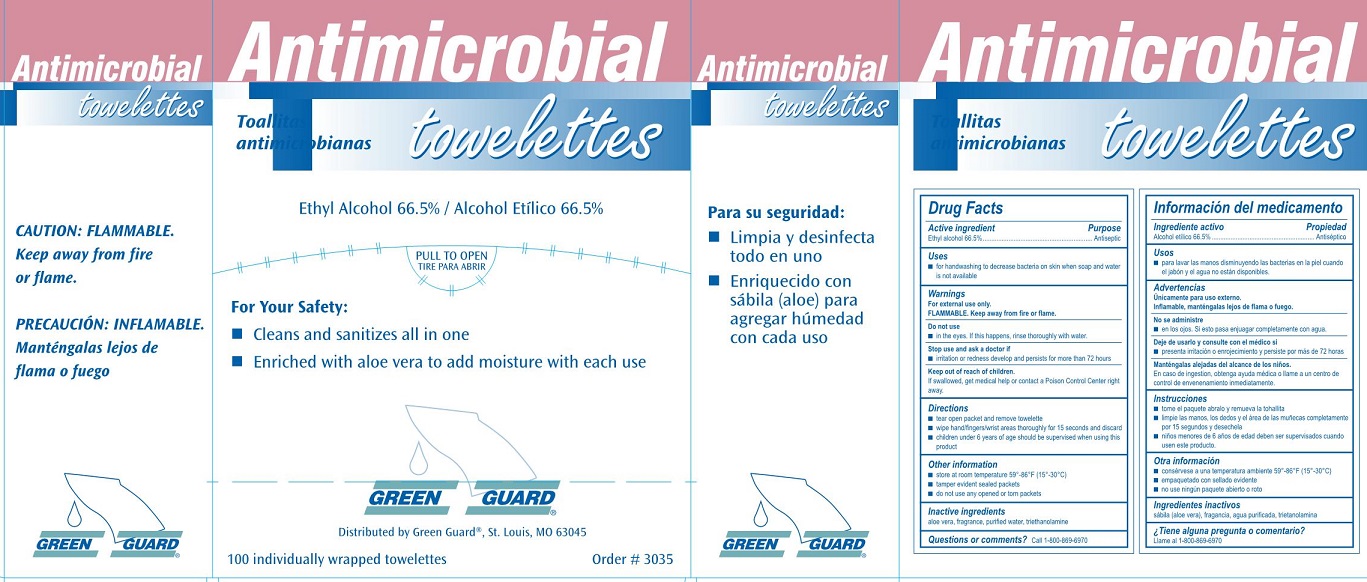
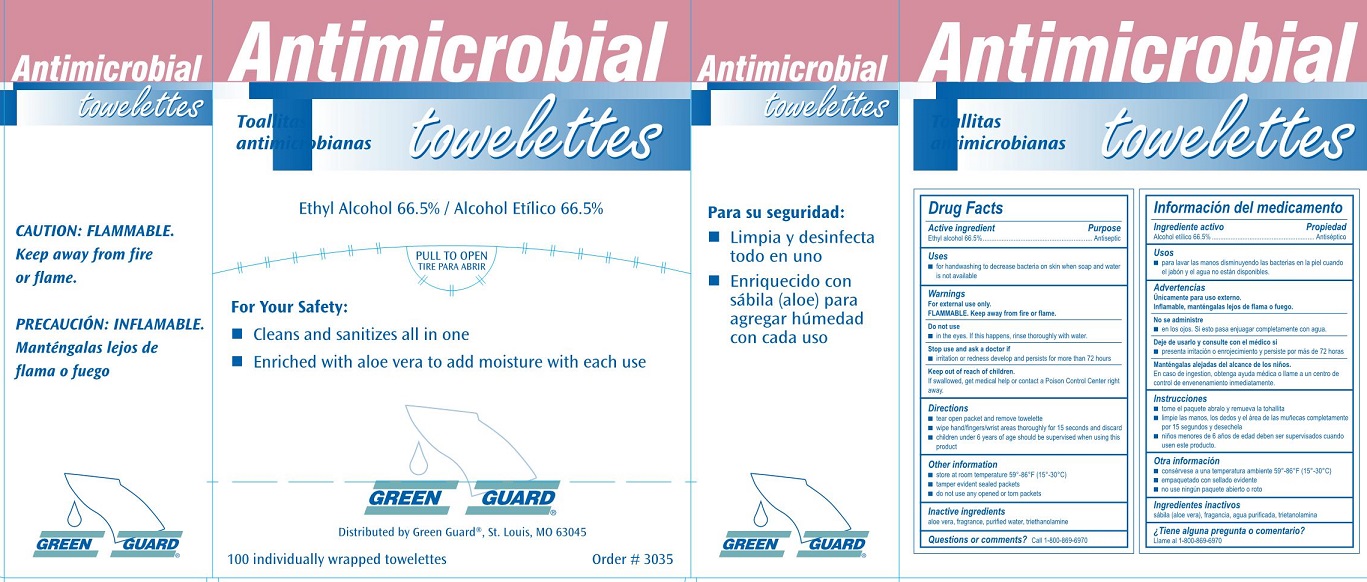
Label: GREEN GUARD ANTIMICROBIAL TOWELETTES- ethyl alcohol 66.5% liquid
- NDC Code(s): 47682-350-33, 47682-350-99
- Packager: Unifirst First Aid Corporation
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 16, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
- Warnings
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Question or comments?
-
Green Guard Antimicrobial Towellettes Label
Antimicrobial
towellettes
Toallitas antimicrobianas
Ethyl Alcohol 66.5% / Alcohol Etilico 66.5%
Pull To Opem
Tire Para Abrir
For Your Safety:
- Cleans and sanitizes all in one
- Enriched with aloe vera to add moisture with each use
GREEN GUARD®
Distributed by Green Guard®, Si. Louis, MO 63045
100 individually wrapped towellettes
Order # 3035
-
INGREDIENTS AND APPEARANCE
GREEN GUARD ANTIMICROBIAL TOWELETTES
ethyl alcohol 66.5% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47682-350 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 665 mL in 1 L Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) ALOE (UNII: V5VD430YW9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47682-350-99 0.0019 L in 1 PACKET; Type 0: Not a Combination Product 06/11/2018 2 NDC:47682-350-33 100 in 1 BOX 06/11/2018 2 0.0019 L in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 06/11/2018 Labeler - Unifirst First Aid Corporation (832947092)