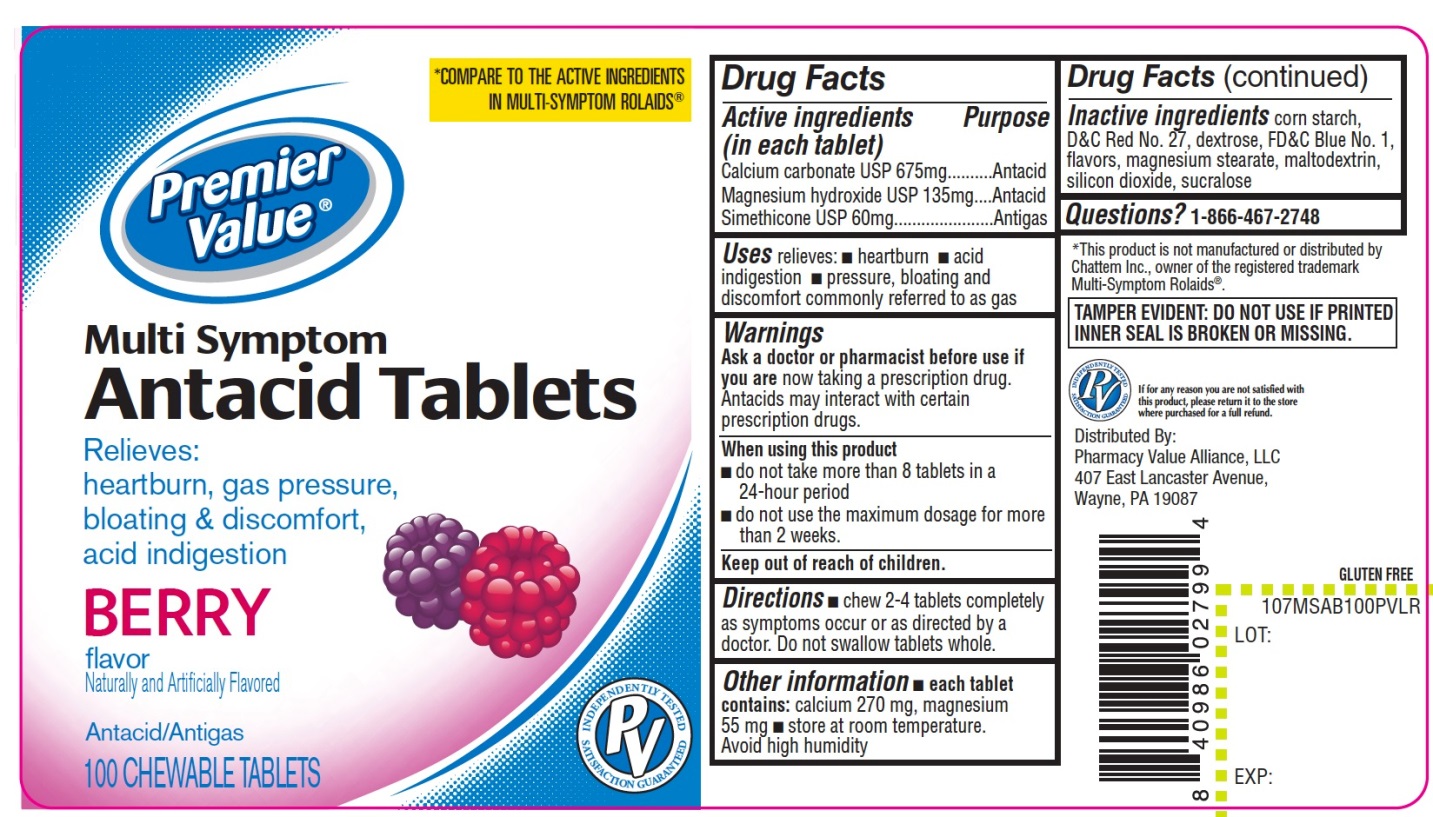
Label: PREMIER VALUE MULTI SYMPTOM ANTACID BERRY FLAVOR- calcium carbonate ,magnesium hydroxide,simethicone tablet, chewable
- NDC Code(s): 68016-679-00
- Packager: Pharmacy Value Alliance LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each tablet)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
NDC# 68016-679-00
*COMPARE TO ACTIVE INGREDIENTS IN MULTI-SYMPTOM ROLAIDS®
Premier Value®
Multi Symptom
Antacid Tablets
Relieves:
heartburn, gas pressure, bloating & discomfort, acid indigestion
BERRY flavor
GLUTEN FREE
Naturally and Artificially Flavored
Antacid/Antigas
K PAREVE
PV
INDEPENDENTLY TESTED
SATISFACTION GURANTEED
100 CHEWABLE TABLETS
If any reason you are not satisfied with this product, please return it in the store where purchased for a full refund.
*This product is not manufactured or distributed by Chattem Inc., owner of the registered trademark Multi-Symptom Rolaids®
TAMPER EVIDENT: DO NOT USE IF PRINTED INNER SEAL IS BROKEN OR MISSING.
DISTRIBUTED BY:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE MULTI SYMPTOM ANTACID BERRY FLAVOR
calcium carbonate ,magnesium hydroxide,simethicone tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-679 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 675 mg MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM HYDROXIDE 135 mg DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 60 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 (UNII: 2LRS185U6K) DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color WHITE (Pinkish) Score no score Shape ROUND Size 16mm Flavor BERRY Imprint Code r7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-679-00 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/20/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 02/20/2019 Labeler - Pharmacy Value Alliance LLC (101668460)