Label: LIBERDOL ANALGESIC- menthol, unspecified form, methyl salicylate liquid
- NDC Code(s): 71262-004-18
- Packager: ViaDerma Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
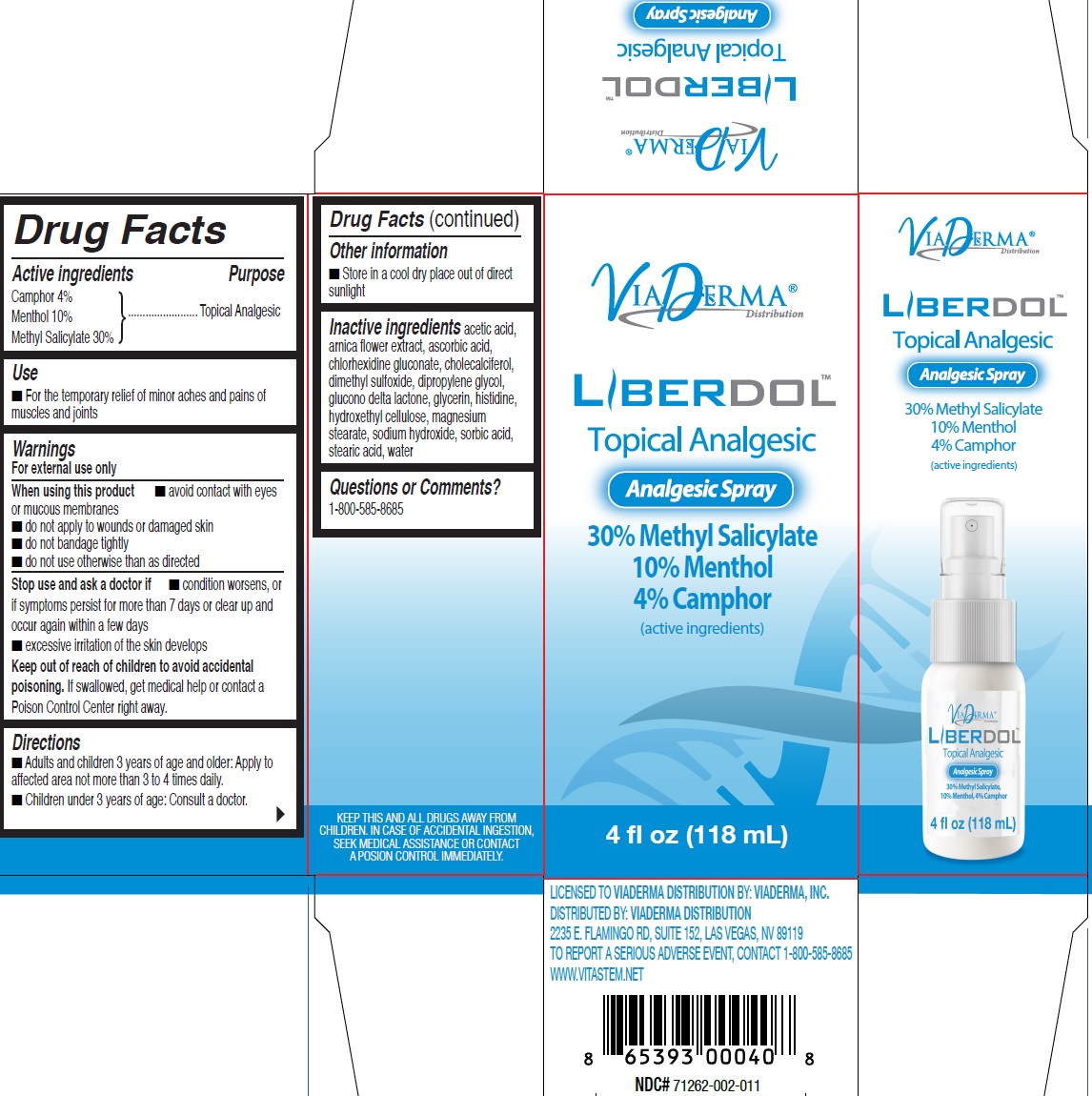
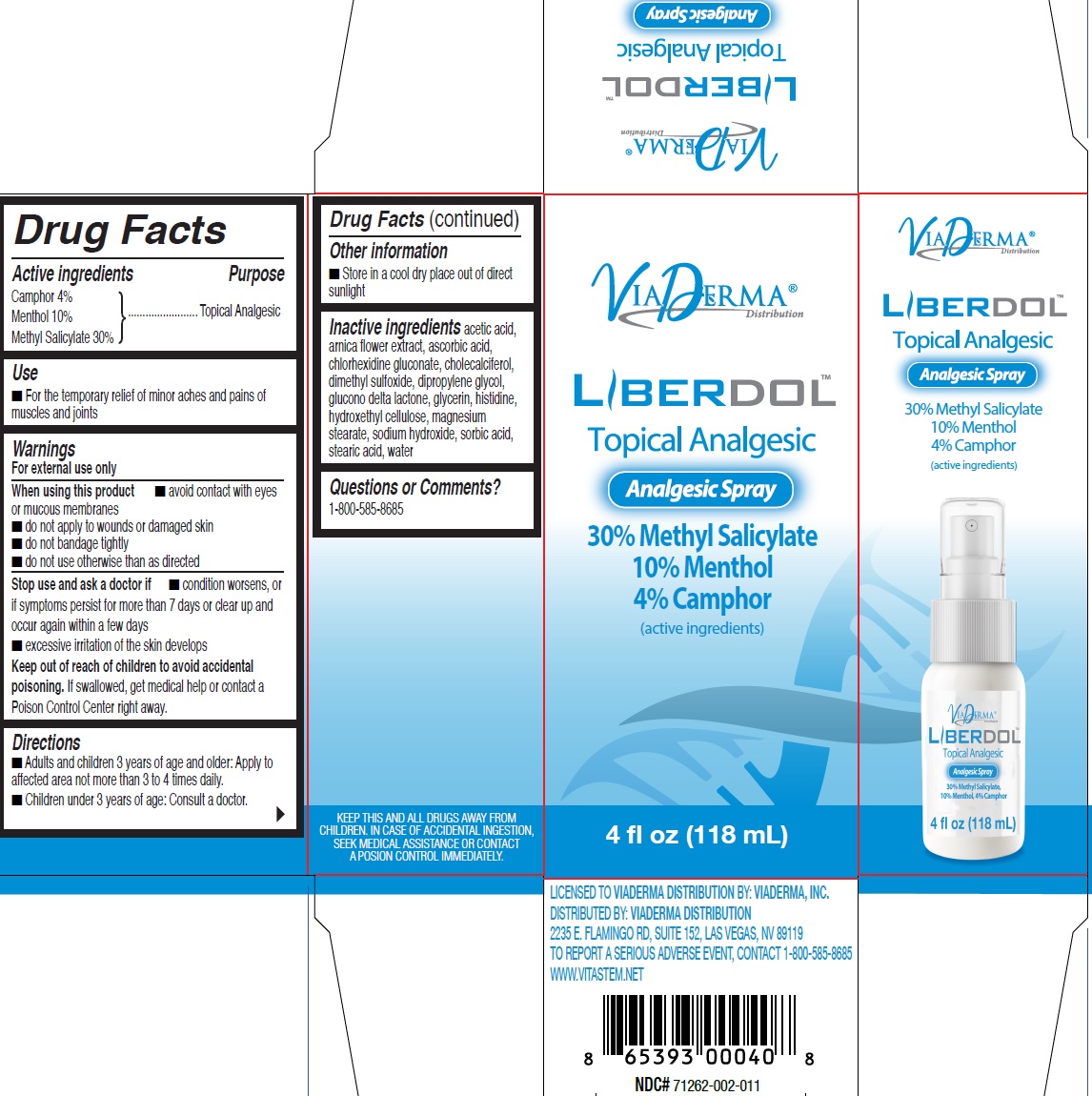
- Drug Facts
- Active ingredients
- Use
-
Warnings
For external use only
When using this product
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged skin
- do not bandage tightly
- do not use otherwise than as directed
- Directions
- Other information
- Inactive ingredients
- Questions or Comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
LIBERDOL ANALGESIC
menthol, unspecified form, methyl salicylate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71262-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 40 mg in 1 mL MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 100 mg in 1 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) CHOLECALCIFEROL (UNII: 1C6V77QF41) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) DIPROPYLENE GLYCOL (UNII: E107L85C40) GLUCONOLACTONE (UNII: WQ29KQ9POT) GLYCERIN (UNII: PDC6A3C0OX) HISTIDINE (UNII: 4QD397987E) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM HYDROXIDE (UNII: 55X04QC32I) SORBIC ACID (UNII: X045WJ989B) STEARIC ACID (UNII: 4ELV7Z65AP) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71262-004-18 118 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 10/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/01/2019 Labeler - ViaDerma Distribution, Inc. (081113521)