Label: EVE LOM RADIANCE PERFECTED TINTED MOISTURISER SPF 15- titanium dioxide and octinoxate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 61601-1193-9, 61601-1194-8, 61601-1195-8, 61601-1196-7 - Packager: Space Brands Limited
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 28, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
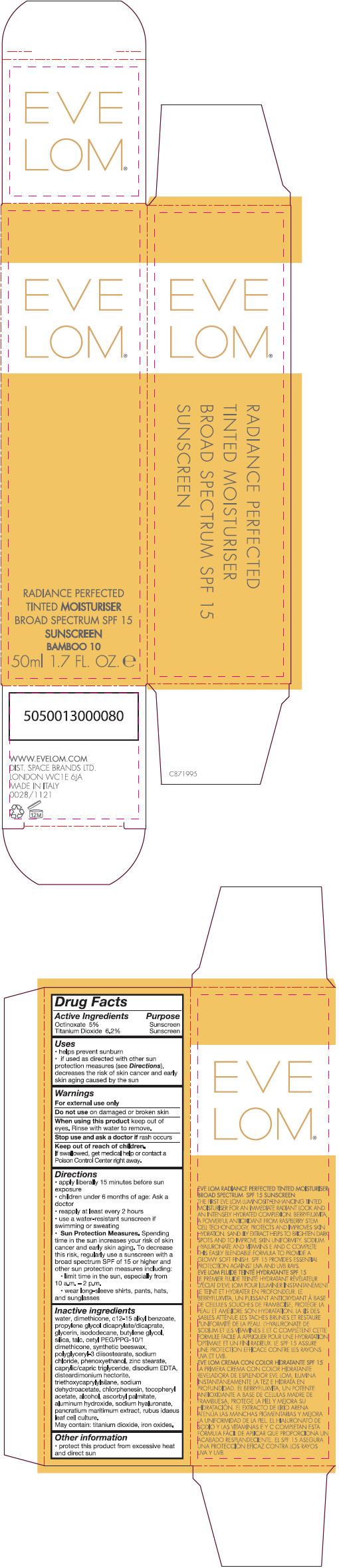
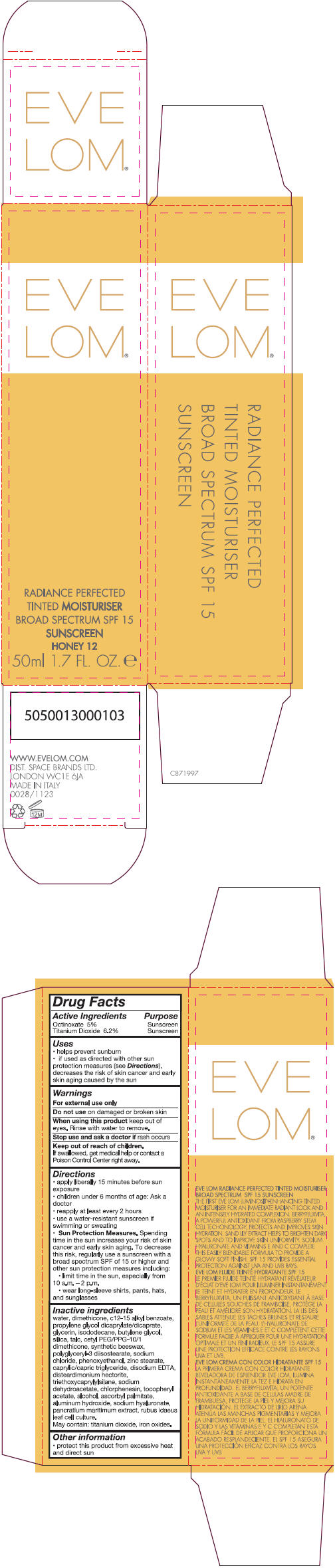
Uses
- helps prevent sunburn
- if used as directed with other sun protection measure (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- children under 6 months of age: Ask a doctor
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
-
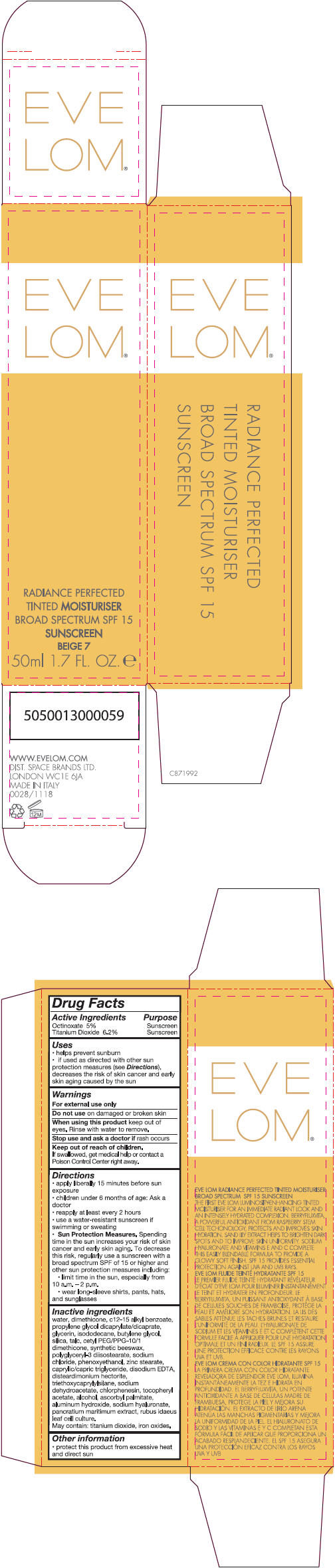
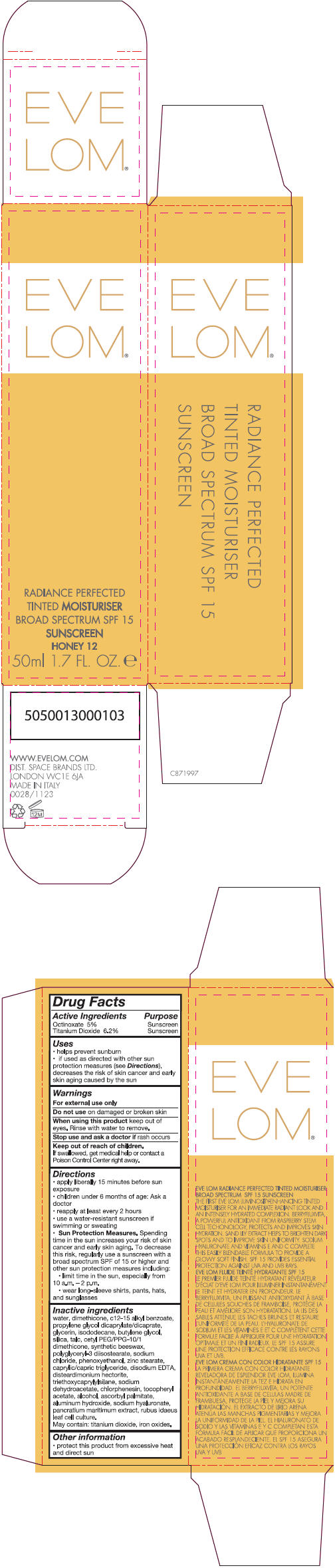
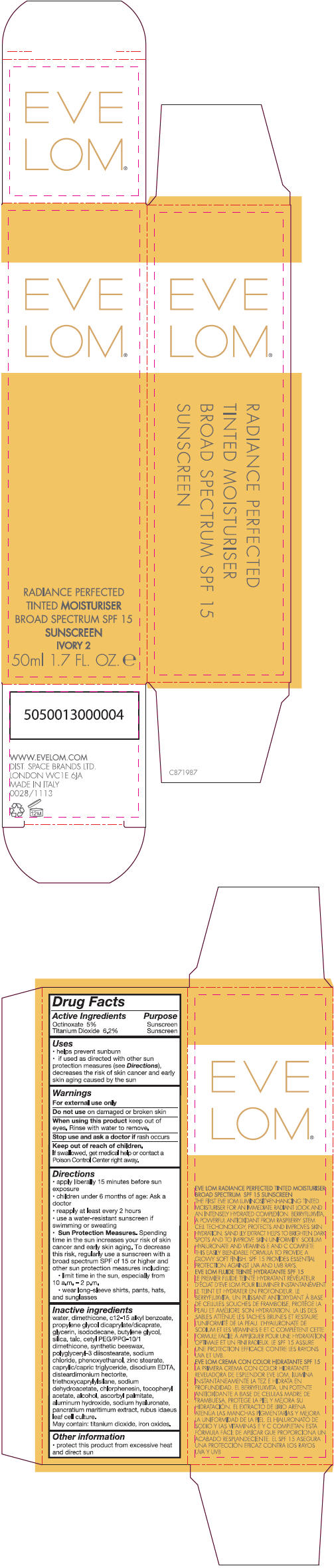
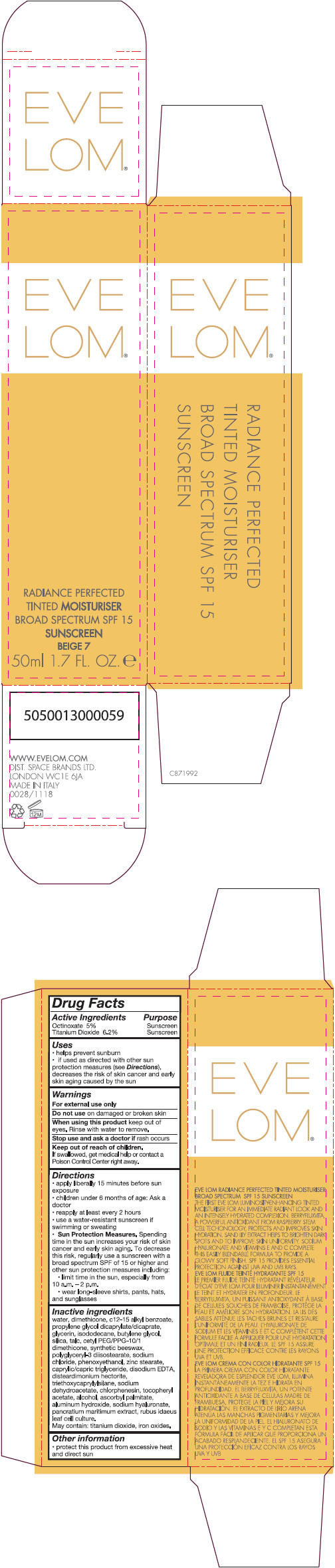
Inactive ingredients
water, dimethicone, c 12-15 alkly benzoate, propylene glycol dicaprylate/dicaprate, glycerin, isododecane, butylene glycol, silica, talc, cetyl PEG/PPG-10/1 dimethicone, synthetic beeswax, polyglyceryl-3 diisostearate, sodium chloride, phenoxyethanol, zinc stearate, caprylic/capric triglyceride, disodium EDTA, disteardimonium hectorite, triethoxycaprylylsilane, sodium dehydroacetate, chlorphenesin, tocopheryl acetate, alcohol, ascorbyl palmitate, aluminum hydroxide, sodium hyaluronate, pancratium maritimum extract, rubus idaeus leaf cell culture.
May contain: titanium dioxide, iron oxides.
- Other information
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - Ivory 2
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - Beige 7
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - Bamboo 10
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton - Honey 12
-
INGREDIENTS AND APPEARANCE
EVE LOM RADIANCE PERFECTED TINTED MOISTURISER SPF 15
titanium dioxide and octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61601-1193 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.1 g in 50 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 2.5 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Dimethicone (UNII: 92RU3N3Y1O) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Propylene Glycol Dicaprylate/Dicaprate (UNII: O4446S9CRA) Glycerin (UNII: PDC6A3C0OX) Isododecane (UNII: A8289P68Y2) Butylene Glycol (UNII: 3XUS85K0RA) Silicon Dioxide (UNII: ETJ7Z6XBU4) Talc (UNII: 7SEV7J4R1U) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Polyglyceryl-3 Diisostearate (UNII: 46P231IQV8) Sodium Chloride (UNII: 451W47IQ8X) Ferric Oxide Yellow (UNII: EX438O2MRT) Phenoxyethanol (UNII: HIE492ZZ3T) Zinc Stearate (UNII: H92E6QA4FV) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Edetate Sodium (UNII: MP1J8420LU) Disteardimonium Hectorite (UNII: X687XDK09L) Triethoxycaprylylsilane (UNII: LDC331P08E) Sodium Dehydroacetate (UNII: 8W46YN971G) Chlorphenesin (UNII: I670DAL4SZ) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Alcohol (UNII: 3K9958V90M) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Ferric Oxide Red (UNII: 1K09F3G675) Ascorbyl Palmitate (UNII: QN83US2B0N) Ferrosoferric Oxide (UNII: XM0M87F357) Hyaluronate Sodium (UNII: YSE9PPT4TH) Product Characteristics Color WHITE (Ivory 2) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61601-1193-9 1 in 1 CARTON 1 2 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/28/2014 EVE LOM RADIANCE PERFECTED TINTED MOISTURISER SPF 15
titanium dioxide and octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61601-1194 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.1 g in 50 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 2.5 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Dimethicone (UNII: 92RU3N3Y1O) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Propylene Glycol Dicaprylate/Dicaprate (UNII: O4446S9CRA) Glycerin (UNII: PDC6A3C0OX) Isododecane (UNII: A8289P68Y2) Butylene Glycol (UNII: 3XUS85K0RA) Silicon Dioxide (UNII: ETJ7Z6XBU4) Talc (UNII: 7SEV7J4R1U) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Polyglyceryl-3 Diisostearate (UNII: 46P231IQV8) Sodium Chloride (UNII: 451W47IQ8X) Ferric Oxide Yellow (UNII: EX438O2MRT) Phenoxyethanol (UNII: HIE492ZZ3T) Zinc Stearate (UNII: H92E6QA4FV) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Edetate Sodium (UNII: MP1J8420LU) Disteardimonium Hectorite (UNII: X687XDK09L) Triethoxycaprylylsilane (UNII: LDC331P08E) Sodium Dehydroacetate (UNII: 8W46YN971G) Chlorphenesin (UNII: I670DAL4SZ) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Alcohol (UNII: 3K9958V90M) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Ferric Oxide Red (UNII: 1K09F3G675) Ascorbyl Palmitate (UNII: QN83US2B0N) Ferrosoferric Oxide (UNII: XM0M87F357) Hyaluronate Sodium (UNII: YSE9PPT4TH) Product Characteristics Color BROWN (Biege 7) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61601-1194-8 1 in 1 CARTON 1 2 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/28/2014 EVE LOM RADIANCE PERFECTED TINTED MOISTURISER SPF 15
titanium dioxide and octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61601-1195 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.1 g in 50 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 2.5 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Dimethicone (UNII: 92RU3N3Y1O) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Propylene Glycol Dicaprylate/Dicaprate (UNII: O4446S9CRA) Glycerin (UNII: PDC6A3C0OX) Isododecane (UNII: A8289P68Y2) Butylene Glycol (UNII: 3XUS85K0RA) Silicon Dioxide (UNII: ETJ7Z6XBU4) Talc (UNII: 7SEV7J4R1U) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Polyglyceryl-3 Diisostearate (UNII: 46P231IQV8) Sodium Chloride (UNII: 451W47IQ8X) Ferric Oxide Yellow (UNII: EX438O2MRT) Phenoxyethanol (UNII: HIE492ZZ3T) Zinc Stearate (UNII: H92E6QA4FV) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Edetate Sodium (UNII: MP1J8420LU) Disteardimonium Hectorite (UNII: X687XDK09L) Triethoxycaprylylsilane (UNII: LDC331P08E) Sodium Dehydroacetate (UNII: 8W46YN971G) Chlorphenesin (UNII: I670DAL4SZ) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Alcohol (UNII: 3K9958V90M) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Ferric Oxide Red (UNII: 1K09F3G675) Ascorbyl Palmitate (UNII: QN83US2B0N) Ferrosoferric Oxide (UNII: XM0M87F357) Hyaluronate Sodium (UNII: YSE9PPT4TH) Product Characteristics Color BROWN (Bamboo 10) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61601-1195-8 1 in 1 CARTON 1 2 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/28/2014 EVE LOM RADIANCE PERFECTED TINTED MOISTURISER SPF 15
titanium dioxide and octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61601-1196 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 3.1 g in 50 mL Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 2.5 g in 50 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Dimethicone (UNII: 92RU3N3Y1O) Alkyl (C12-15) Benzoate (UNII: A9EJ3J61HQ) Propylene Glycol Dicaprylate/Dicaprate (UNII: O4446S9CRA) Glycerin (UNII: PDC6A3C0OX) Isododecane (UNII: A8289P68Y2) Butylene Glycol (UNII: 3XUS85K0RA) Silicon Dioxide (UNII: ETJ7Z6XBU4) Talc (UNII: 7SEV7J4R1U) Cetyl PEG/PPG-10/1 Dimethicone (HLB 2) (UNII: V2W71V8T0X) Polyglyceryl-3 Diisostearate (UNII: 46P231IQV8) Sodium Chloride (UNII: 451W47IQ8X) Ferric Oxide Yellow (UNII: EX438O2MRT) Phenoxyethanol (UNII: HIE492ZZ3T) Zinc Stearate (UNII: H92E6QA4FV) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Edetate Sodium (UNII: MP1J8420LU) Disteardimonium Hectorite (UNII: X687XDK09L) Triethoxycaprylylsilane (UNII: LDC331P08E) Sodium Dehydroacetate (UNII: 8W46YN971G) Chlorphenesin (UNII: I670DAL4SZ) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Alcohol (UNII: 3K9958V90M) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Ferric Oxide Red (UNII: 1K09F3G675) Ascorbyl Palmitate (UNII: QN83US2B0N) Ferrosoferric Oxide (UNII: XM0M87F357) Hyaluronate Sodium (UNII: YSE9PPT4TH) Product Characteristics Color BROWN (Honey 12) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61601-1196-7 1 in 1 CARTON 1 2 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 02/28/2014 Labeler - Space Brands Limited (218214381) Establishment Name Address ID/FEI Business Operations Intercos Europe S.p.A. 438961310 MANUFACTURE(61601-1193, 61601-1194, 61601-1195, 61601-1196)