Label: LEVOMEFOLATE CALCIUM-PYRIDOXAL PHOSPHATE-MECOBALAMIN- levomefolate calcium, pyridoxal phosphate, and methylcobalamin tablet, coated
- NHRIC Code(s): 69367-282-09
- Packager: Westminster Pharmaceuticals, LLC
- Category: MEDICAL FOOD
- DEA Schedule: None
- Marketing Status: MEDICAL FOOD
Drug Label Information
Updated October 4, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
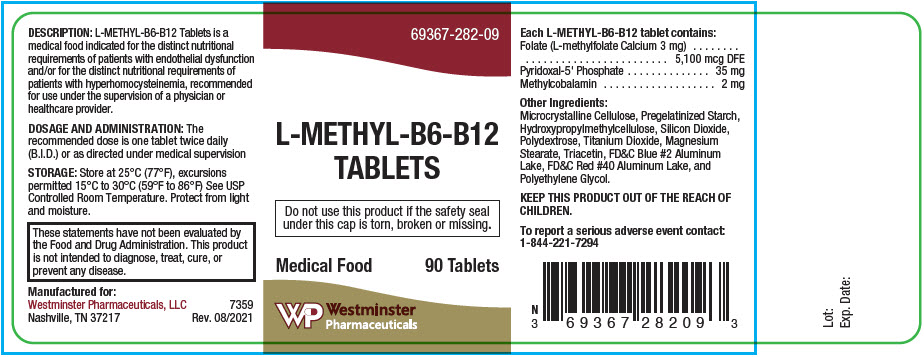
DESCRIPTION
Each round, coated purple colored tablet contains:
Folate (L-methylfolate Calcium 3 mg) 5,100 mcg DFE Pyridoxal-5'-Phosphate 35 mg Methylcobalamin 2 mg Other Ingredients: Microcrystalline Cellulose, Pregelatinized Starch, Hydroxypropyl Methylcellulose, Silicon Dioxide, Polydextrose, Titanium Dioxide, Magnesium Stearate, Triacetin, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, and Polyethylene Glycol.
L-Methyl-B6-B12 Tablets do not contain sugar, lactose, yeast or gluten.
L-Methyl-B6-B12 Tablets is labeled as a medical food for use under the active and ongoing medical supervision of a physician or health-care provider on a recurring basis for, among other things, instructions on their use.
Medical foods are intended for the dietary management of a patient who, because of therapeutic or chronic medical needs, has limited or impaired capacity to ingest, digest, absorb or metabolize ordinary foodstuffs or certain nutrients, or who has other special medically determined nutrient requirements, the dietary management of which cannot be achieved by the modification of the normal diet alone. This Medical Food is not subject to NDA or ANDA approval and is not an Orange Book product. Although FDA does not require a prescription for Medical Foods, this product is intended to be used under active medical supervision. This product is not eligible for government reimbursement under federal programs, but is eligible for reimbursement under state programs on a case-by-case basis. Please check with a specific state to determine proper reimbursement eligibility.
These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. - INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
Folic acid, when administered as a single agent in doses above 0.1 mg daily, may obscure the detection of B12 deficiency. Folate therapy alone may be inadequate for the treatment of a B12 deficiency.
Patient Information
L-Methyl-B6-B12 Tablets is a medical food1 to be used only under medical supervision of a physician or health-care provider.
DRUG INTERACTIONS
L-Methyl-B6-B12 Tablets added to other Drugs
High dose folic acid may result in decreased serum levels for pyrimethamine and first-generation anticonvulsants (carbamazepine, fosphenytoin, phenytoin, phenobarbital, primidone, valproic acid, valproate).2,3 This may possibly reduce first generation anticonvulsants effectiveness and/or increase the frequency of seizures in susceptible patients.2,3 While the concurrent use of folic acid and first generation anticonvulsants or pyrimethamine may result in decreased efficacy of anticonvulsants, no such decreased effectiveness has been reported with the use of L-methylfolate. Nevertheless, caution should be used when prescribing L-Methyl-B6-B12 Tablets among patients who are receiving treatment with first generation anticonvulsants or pyrimethamine. Pyridoxal 5'-phosphate should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxal 5'-phosphate. However, pyridoxal 5'-phosphate may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa.
Capecitabine (Xeloda®) toxicity may increase with the addition of leucovorin (5-formyltetrahydrofolate) (folate).
Drugs added to L-Methyl-B6-B12 Tablets
Antibiotics may alter the intestinal microflora and may decrease the absorption of methylcobalamin. Cholestyramine, colchicines or colestipol may decrease the enterohepatic re-absorption of methylcobalamin. Metformin, para-aminosalicylic acid and potassium chloride may decrease the absorption of methylcobalamin. Nitrous oxide can produce a functional methylcobalamin deficiency. Several drugs are associated with lowering serum folate levels or reducing the amount of active folate available. First generation anticonvulsants (carbamazepine, fosphenytoin, phenytoin, phenobarbital, primidone, valproic acid, valproate)2,3 and lamotrigine4 (a second-generation anticonvulsant) may decrease folate plasma levels. Information on other second-generation anticonvulsants impact on folate levels is limited and cannot be ruled out.
Divalproex sodium,5 topiramate,6 gabapentin,7 pregabalin,8 levetiracetam,9 tiagabine,10 zonisamide,11 have not reported the potential to lower folate in their respective prescribing information. Methotrexate, alcohol (in excess), sulfasalazine, cholestyramine, colchicine, colestipol, L-dopa, methylprednisone, NSAIDs (high dose), pancreatic enzymes (pancrelipase, pancreatin), pentamidine, pyrimethamine, smoking, triamterene, and trimethoprim may decrease folate plasma levels. Warfarin can produce significant impairment in folate status after a 6-month therapy.
-
ADVERSE REACTIONS
Allergic reactions have been reported following the use of oral L-methylfolate Calcium.12 Acne, skin reactions, allergic reactions, photosensitivity, nausea, vomiting, abdominal pain, loss of appetite, increased liver function test results, paresthesia, somnolence, nausea and headaches have been reported with pyridoxal 5'-phosphate.13 Mild transient diarrhea, polycythemia vera, itching, transitory exanthema and the feeling of swelling of the entire body has been associated with methylcobalamin.
Keep this and all medications out of the reach of children. In case of accidental overdose, seek professional assistance or contact a poison control center immediately.
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
L-Methyl-B6-B12 Tablets is available as a round, coated purple-colored tablet. Debossed with "282" on one side and plain on the other.
Bottles of 90 tablets Product Code 69367-282-091 Use under supervision of a physician or health-care provider.
- 1
- Westminster Pharmaceuticals, LLC does not represent this product code to be a National Drug Code (NDC) number. Product Codes are formatted according to standard industry practice, to meet the formatting requirements of pharmacy and health insurance computer systems.
-
REFERENCES
1 United States Food and Drug Administration Title 21 Code of federal Regulations 101.9(j)(8).
2 PDR® For Nutritional Supplements, 2001; ISBN: 1-56363-364-7:157-167.
3 Leucovorin Calcium (folinic acid) For Injection Prescribing Information: December 2003; Mayne Pharma (USA) Inc.
4 Lamictal® (lamotrigine) Prescribing Information: August 2005; GlaxoSmith-Kline.
5 Depakote® (divalproex sodium) Prescribing Information: January 2006; Abbott Laboratories.
6 Topamax® (topiramate) Prescribing Information: June 2005; ORTHO-McNEIL NEUROLOGICS, INC.
7 Neurontin® (gabapentin) Prescribing Information: December 2005; Parke-Davis.
8 Lyrica® (pregabalin) Prescribing Information: March 2006; Parke-Davis.
9 Keppra® (levetiracetam) Prescribing Information: March 2007; UCB, Inc.
10 Gabitril® (tiagabine) Prescribing Information: March 2005: Cephalon, Inc.
11 Zonegran® (zonisamide) Prescribing Information: December 2004: Elan Pharma International Ltd.; licensed to Eisai Inc.
12 Natural Standard Research Collaboration (NIH). Folate (folic acid) Monograph 2009.
13 Alternative Medicine Review Vitamin B6 Monograph Volume 6, Number 1, 2001.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
LEVOMEFOLATE CALCIUM-PYRIDOXAL PHOSPHATE-MECOBALAMIN
levomefolate calcium, pyridoxal phosphate, and methylcobalamin tablet, coatedProduct Information Product Type MEDICAL FOOD Item Code (Source) NHRIC:69367-282 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 3 mg PYRIDOXAL PHOSPHATE (UNII: 5V5IOJ8338) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 35 mg METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 2 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM STEARATE (UNII: 70097M6I30) TRIACETIN (UNII: XHX3C3X673) FD&C BLUE NO. 2 ALUMINUM LAKE (UNII: 4AQJ3LG584) FD&C RED NO. 40 (UNII: WZB9127XOA) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Product Characteristics Color PURPLE Score no score Shape ROUND Size 10mm Flavor Imprint Code 282 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69367-282-09 90 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date MEDICAL FOOD 04/09/2021 07/01/2024 Labeler - Westminster Pharmaceuticals, LLC (079516651) Establishment Name Address ID/FEI Business Operations LGM Pharma Solutions, LLC 117549200 MANUFACTURE(69367-282)