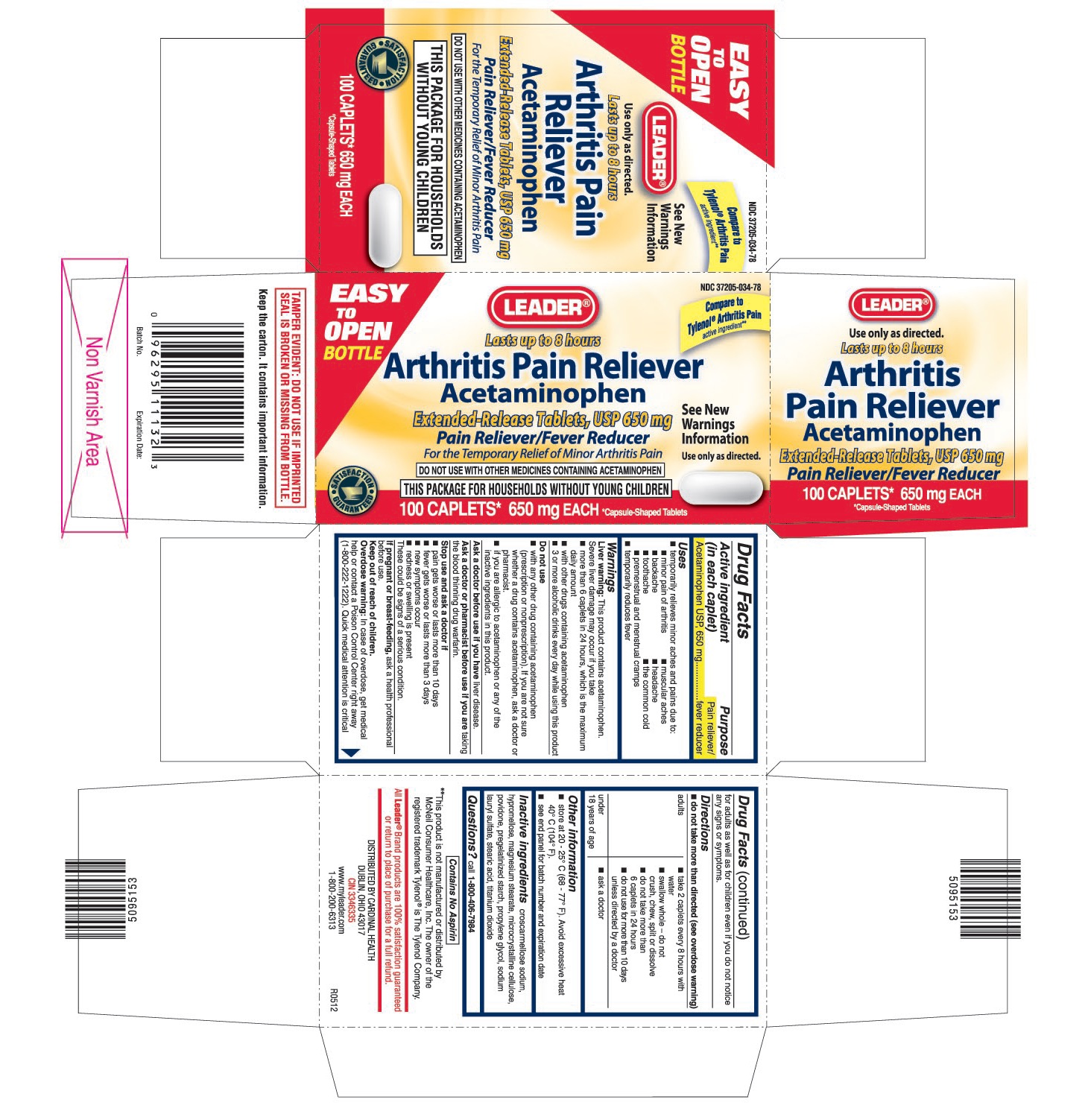
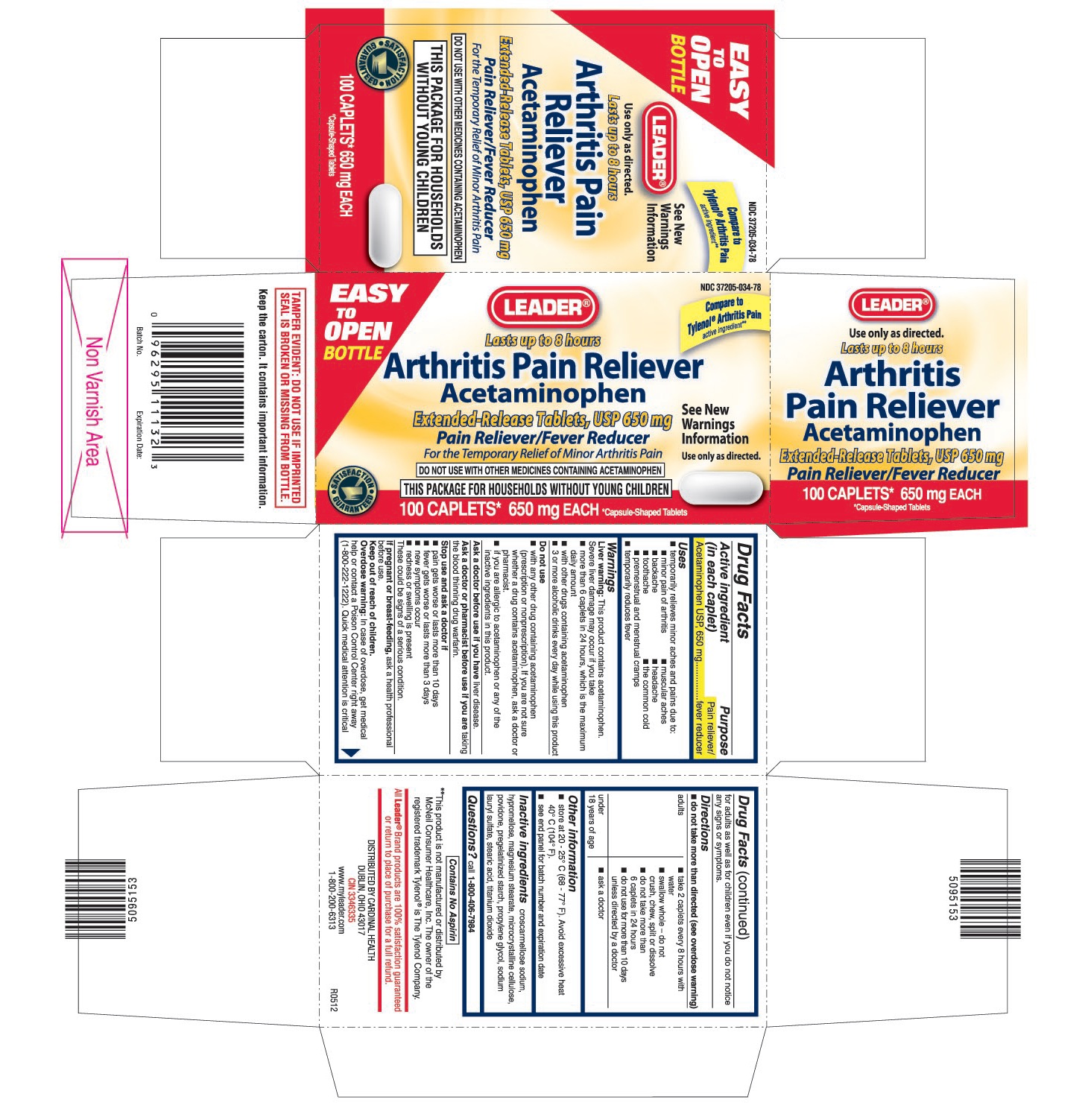
Label: LEADER ARTHRITIS PAIN RELIEVER- acetaminophen tablet, film coated, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 37205-034-71, 37205-034-78 - Packager: Cardinal Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 16, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT (IN EACH CAPLET)
- PURPOSE
- USES
-
WARNINGS
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
PRINCIPAL DISPLAY PANEL
Acetaminophen Extended-Release Tablets, USP 650 mg
For the Temporary Relief of Minor Arthritis Pain
DO NOT USE WITH OTHER MEDICINES CONTAINING ACETAMINOPHEN
THIS PACKAGE FOR HOUSEHOLDS WITHOUT YOUNG CHILDREN
Compare to Tylenol® Arthritis Pain active ingredient**
**This product is not manufactured or distributed by McNeil Consumer Healthcare, Inc. The owner of the registered trademark Tylenol® is The Tylenol Company.
-
INGREDIENTS AND APPEARANCE
LEADER ARTHRITIS PAIN RELIEVER
acetaminophen tablet, film coated, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37205-034 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) POVIDONE (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white Score no score Shape OVAL (capsule shaped) Size 19mm Flavor Imprint Code cor116 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37205-034-71 50 in 1 BOTTLE 2 NDC:37205-034-78 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076200 04/30/2002 Labeler - Cardinal Health (097537435) Registrant - Ohm Laboratories Inc. (051565745) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 manufacture