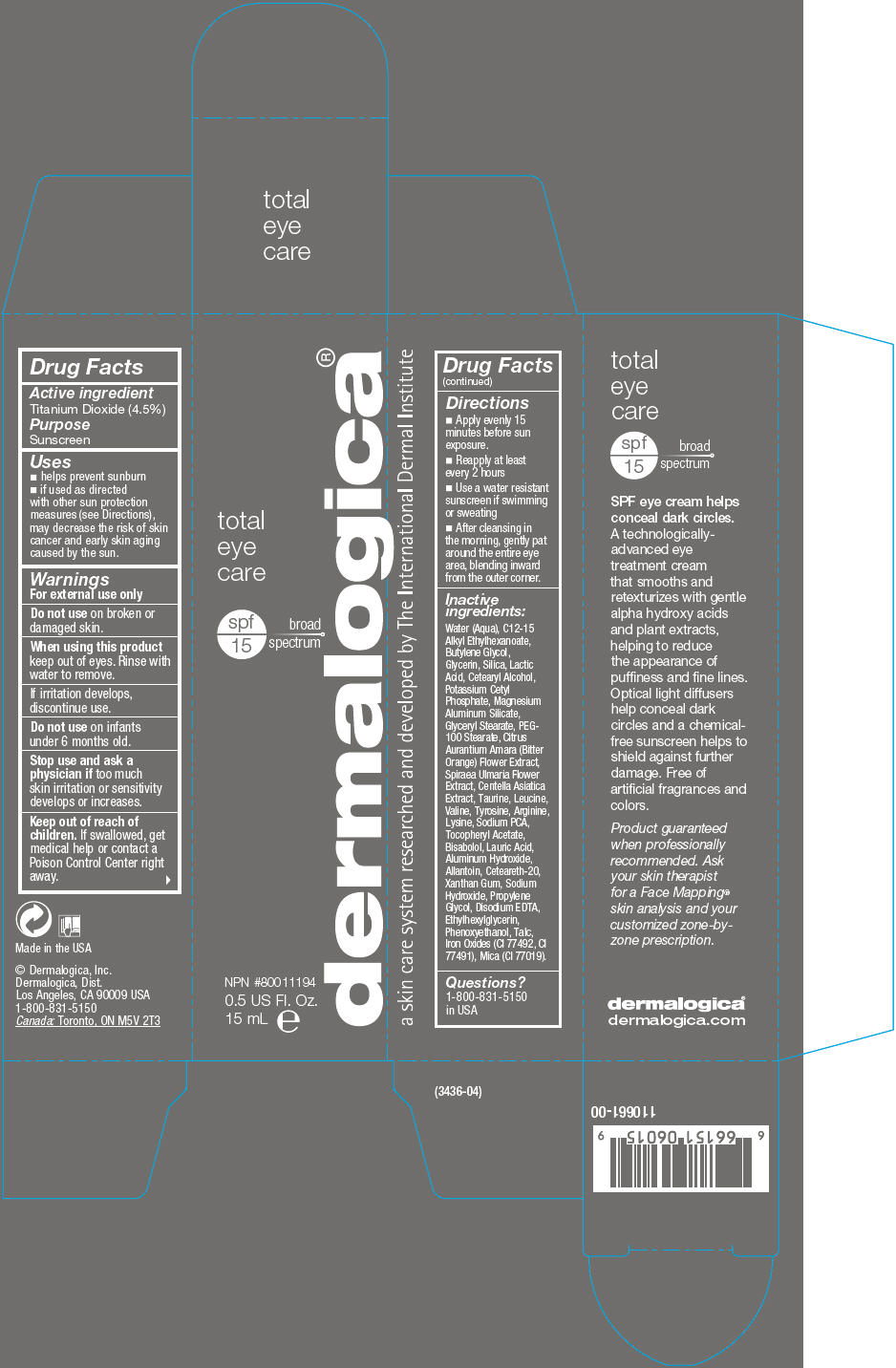
Label: TOTAL EYE CARE SPF 15- titanium dioxide lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 68479-360-00, 68479-360-01, 68479-360-02 - Packager: Dermalogica
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 23, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), may decrease the risk of skin cancer and early skin aging caused by the sun.
- Warnings
- Directions
-
Inactive ingredients
Water (Aqua), C12-15 Alkyl Ethylhexanoate, Butylene Glycol, Glycerin, Silica, Lactic Acid, Cetearyl Alcohol, Potassium Cetyl Phosphate, Magnesium Aluminum Silicate, Glyceryl Stearate, PEG-100 Stearate, Citrus Aurantium Amara (Bitter Orange) Flower Extract, Spiraea Ulmaria Flower Extract, Centella Asiatica Extract, Taurine, Leucine, Valine, Tyrosine, Arginine, Lysine, Sodium PCA, Tocopheryl Acetate, Bisabolol, Lauric Acid, Aluminum Hydroxide, Allantoin, Ceteareth-20, Xanthan Gum, Sodium Hydroxide, Propylene Glycol, Disodium EDTA, Ethylhexylglycerin, Phenoxyethanol, Talc, Iron Oxides (CI 77492, CI 77491), Mica (CI 77019).
- Questions?
- PRINCIPAL DISPLAY PANEL - 15 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
TOTAL EYE CARE SPF 15
titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68479-360 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) Glycerin (UNII: PDC6A3C0OX) Silicon Dioxide (UNII: ETJ7Z6XBU4) Lactic Acid (UNII: 33X04XA5AT) Cetostearyl Alcohol (UNII: 2DMT128M1S) Potassium Cetyl Phosphate (UNII: 03KCY6P7UT) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Glyceryl Monostearate (UNII: 230OU9XXE4) PEG-100 Stearate (UNII: YD01N1999R) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Citrus Aurantium Flower (UNII: O730ZX2Z83) Phenoxyethanol (UNII: HIE492ZZ3T) Propylene Glycol (UNII: 6DC9Q167V3) Sodium Pyrrolidone Carboxylate (UNII: 469OTG57A2) Filipendula Ulmaria Flower (UNII: 06L18L32G6) Centella Asiatica (UNII: 7M867G6T1U) Sodium Hydroxide (UNII: 55X04QC32I) Aluminum Hydroxide (UNII: 5QB0T2IUN0) Lysine (UNII: K3Z4F929H6) Xanthan Gum (UNII: TTV12P4NEE) Levomenol (UNII: 24WE03BX2T) Arginine (UNII: 94ZLA3W45F) Lauric Acid (UNII: 1160N9NU9U) Edetate Disodium (UNII: 7FLD91C86K) Taurine (UNII: 1EQV5MLY3D) Tyrosine (UNII: 42HK56048U) Ethylhexylglycerin (UNII: 147D247K3P) .Alpha.-Tocopherol Acetate (UNII: 9E8X80D2L0) Allantoin (UNII: 344S277G0Z) Leucine (UNII: GMW67QNF9C) Valine (UNII: HG18B9YRS7) Talc (UNII: 7SEV7J4R1U) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BARIUM CARBONATE (UNII: 6P669D8HQ8) FERRIC OXIDE RED (UNII: 1K09F3G675) Product Characteristics Color PINK Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68479-360-02 1 in 1 CARTON 1 15 mL in 1 TUBE 2 NDC:68479-360-01 4 mL in 1 TUBE 3 NDC:68479-360-00 2 mL in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/29/2012 Labeler - Dermalogica (177698560) Establishment Name Address ID/FEI Business Operations PakLab 177711082 MANUFACTURE(68479-360) Establishment Name Address ID/FEI Business Operations Diamond Wipes 161104729 MANUFACTURE(68479-360)