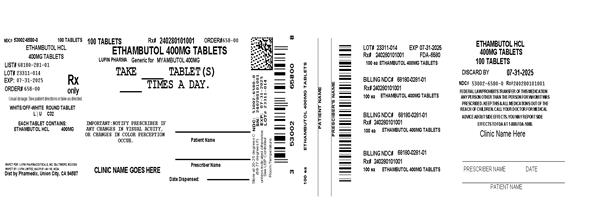
Label: ETHAMBUTOL HYDROCHLORIDE tablet
- NDC Code(s): 53002-6580-0
- Packager: RPK Pharmaceuticals, Inc.
- This is a repackaged label.
- Source NDC Code(s): 68180-281
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis. Ethambutol hydrochloride is a white, crystalline powder, It is freely soluble in water; soluble in alcohol and in methanol. The structural formula is:
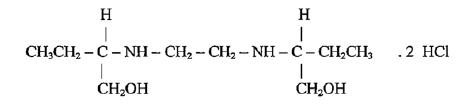
C10H24N2O2•2HCl M.W. 277.23
(+)-2,2’ (Ethylenediimino)-di-1-butanol dihydrochloride
Each tablet, for oral administration, contains 100 mg or 400 mg Ethambutol Hydrochloride. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, corn starch, magnesium stearate, povidone and talc. Film coating contains: ethyl cellulose, hypromellose, macrogol, propylene glycol, talc and titanium dioxide.
-
CLINICAL PHARMACOLOGY
Ethambutol hydrochloride, following a single oral dose of 25 mg/kg of body weight, attains a peak of 2 to 5 mcg/mL in serum 2 to 4 hours after administration. When the drug is administered daily for longer periods of time at this dose, serum levels are similar. The serum level of ethambutol falls to undetectable levels by 24 hours after the last dose except in some patients with abnormal renal function. The intercellular concentrations of erythrocytes reach peak values approximately twice those of plasma and maintain this ratio throughout the 24 hours.
During the 24-hour period following oral administration of ethambutol approximately 50 percent of the initial dose is excreted unchanged in the urine, while an additional 8 to 15 percent appears in the form of metabolites. The main path of metabolism appears to be an initial oxidation of the alcohol to an aldehydic intermediate, followed by conversion to a dicarboxylic acid. From 20 to 22 percent of the initial dose is excreted in the feces as unchanged drug. No drug accumulation has been observed with consecutive single daily doses of 25 mg/kg in patients with normal kidney function, although marked accumulation has been demonstrated in patients with renal insufficiency.
Ethambutol diffuses into actively growing mycobacterium cells such as tubercle bacilli. Ethambutol appears to inhibit the synthesis of one or more metabolites, thus causing impairment of cell metabolism, arrest of multiplication, and cell death. No cross resistance with other available antimycobacterial agents has been demonstrated.
Ethambutol has been shown to be effective against strains of Mycobacterium tuberculosis but does not seem to be active against fungi, viruses, or other bacteria. Mycobacterium tuberculosis strains previously unexposed to ethambutol have been uniformly sensitive to concentrations of 8 or less mcg/ mL, depending on the nature of the culture media. When ethambutol has been used alone for treatment of tuberculosis, tubercle bacilli from these patients have developed resistance to ethambutol hydrochloride by in-vitro susceptibility tests; the development of resistance has been unpredictable and appears to occur in a step-like manner. No cross resistance between ethambutol and other antituberculous drugs has been reported. Ethambutol has reduced the incidence of the emergence of mycobacterial resistance to isoniazid when both drugs have been used concurrently. An agar diffusion microbiologic assay, based upon inhibition of Mycobacterium smegmatis (ATCC 607) may be used to determine concentrations of ethambutol in serum and urines.
ANIMAL PHARMACOLOGY
Toxicological studies in dogs on high prolonged doses produced evidence of myocardial damage and failure, and depigmentation of the tapetum lucidum of the eyes, the significance of which is not known. Degenerative changes in the central nervous system, apparently not dose-related, have also been noted in dogs receiving ethambutol hydrochloride over a prolonged period. In the rhesus monkey, neurological signs appeared after treatment with high doses given daily over a period of several months. These were correlated with specific serum levels of ethambutol and with definite neuroanatomical changes in the central nervous system. Focal interstitial carditis was also noted in monkeys which received ethambutol hydrochloride in high doses for a prolonged period.
-
INDICATIONS AND USAGE
Ethambutol hydrochloride is indicated for the treatment of pulmonary tuberculosis. It should not be used as the sole antituberculous drug, but should be used in conjunction with at least one other antituberculous drug. Selection of the companion drug should be based on clinical experience, considerations of comparative safety and appropriate in-vitro susceptibility studies. In patients who have not received previous antituberculous therapy, i.e., initial treatment, the most frequently used regimens have been the following:
- Ethambutol plus isoniazid
- Ethambutol plus isoniazid plus streptomycin.
In patients who have received previous antituberculous therapy, mycobacterial resistance to other drugs used in initial therapy is frequent. Consequently, in such retreatment patients, ethambutol should be combined with at least one of the second line drugs not previously administered to the patient and to which bacterial susceptibility has been indicated by appropriate in-vitro studies. Antituberculous drugs used with ethambutol have included cycloserine, ethionamide, pyrazinamide, viomycin and other drugs. Isoniazid, aminosalicylic acid, and streptomycin have also been used in multiple drug regimens. Alternating drug regimens have also been utilized.
-
CONTRAINDICATIONS
Ethambutol hydrochloride is contraindicated in patients who are known to be hypersensitive to this drug. It is also contraindicated in patients with known optic neuritis unless clinical judgement determines that it may be used. Ethambutol hydrochloride is contraindicated in patients who are unable to appreciate and report visual side effects or changes in vision (e.g., young children, unconscious patients).
-
WARNINGS
Ethambutol hydrochloride may produce decreases in visual acuity which appear to be due to optic neuritis. This effect may be related to dose and duration of treatment. This effect is generally reversible when administration of the drug is discontinued promptly. However, irreversible blindness has been reported. (See PRECAUTIONSand ADVERSE REACTIONS).
Liver toxicities including fatalities have been reported (see ADVERSE REACTIONS). Baseline and periodic assessment of hepatic function should be performed.
-
PRECAUTIONS
Ethambutol hydrochloride is not recommended for use in pediatric patients under thirteen years of age since safe conditions for use have not been established.
Patients with decreased renal function need the dosage reduced as determined by serum levels of ethambutol, since the main path of excretion of this drug is by the kidneys.
Because this drug may have adverse effects on vision, physical examination should include ophthalmoscopy, finger perimetry and testing of color discrimination. In patients with visual defects such as cataracts, recurrent inflammatory conditions of the eye, optic neuritis, and diabetic retinopathy, the evaluation of changes in visual acuity is more difficult, and care should be taken to be sure the variations in vision are not due to the underlying disease conditions. In such patients, consideration should be given to relationship between benefits expected and possible visual deterioration since evaluation of visual changes is difficult. (For recommended procedures, see next paragraphs under ADVERSE REACTIONS).
As with any potent drug, baseline and periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, should be performed.
Drug Interactions
The results of a study of coadministration of ethambutol hydrochloride (50mg/kg) with an aluminum hydroxide containing antacid to 13 patients with tuberculosis showed a reduction of mean serum concentrations and urinary excretion of ethambutol of approximately 20% and 13%, respectively, suggesting that the oral absorption of ethambutol may be reduced by these antacid products. It is recommended to avoid concurrent administration of ethambutol with aluminum hydroxide containing antacids for at least 4 hours following ethambutol administration.
Pregnancy
Teratogenic Effects: Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. There are reports of ophthalmic abnormalities occurring in infants born to women on antituberculous therapy that included ethambutol hydrochloride. Ethambutol hydrochloride should be used during pregnancy only if the benefit justifies the potential risk to the fetus.
Ethambutol hydrochloride has been shown to be teratogenic in pregnant mice and rabbits when given in high doses. When pregnant mice or rabbits were treated with high doses of ethambutol hydrochloride, fetal mortality was slightly but not significantly (P>0.05) increased. Female rats treated with ethambutol hydrochloride displayed slight but insignificant (P>0.05) decreases in fertility and litter size. In fetuses born of mice treated with high doses of ethambutol hydrochloride during pregnancy, a low incidence of cleft palate, exencephaly and abnormality of the vertebral column were observed. Minor abnormalities of the cervical vertebra were seen in the newborn of rats treated with high doses of ethambutol hydrochloride during pregnancy. Rabbits receiving high doses of ethambutol hydrochloride during pregnancy gave birth to two fetuses with monophthalmia, one with a shortened right forearm accompanied by bilateral wrist-joint contracture and one with hare lip and cleft palate.
Nursing Mothers
Ethambutol hydrochloride is excreted into breast milk. The use of ethambutol hydrochloride should be considered only if the expected benefit to the mother outweighs the potential risk to the infant.
Pediatric Use
Ethambutol hydrochloride is not recommended for use in pediatric patients under thirteen years of age since safe conditions for use have not been established.
Geriatric Use
There are limited data on the use of ethambutol hydrochloride in the elderly. One study of 101 patients, 65 years and older, on multiple drug antituberculosis regimens included 94 patients on ethambutol hydrochloride. No differences in safety or tolerability were observed in these patients compared with that reported in adults in general. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
ADVERSE REACTIONS
Ethambutol hydrochloride may produce decreases in visual acuity, including irreversible blindness, which appear to be due to optic neuritis. Optic neuropathy including optic neuritis or retrobulbar neuritis occurring in association with ethambutol therapy may be characterized by one or more of the following events: decreased visual acuity, scotoma, color blindness, and/or visual defect. These events have also been reported in the absence of a diagnosis of optic or retrobulbar neuritis.
Patients should be advised to report promptly to their physician any change of visual acuity.
The change in visual acuity may be unilateral or bilateral and hence each eye must be tested separately and both eyes tested together. Testing of visual acuity should be performed before beginning ethambutol hydrochloride therapy and periodically during drug administration, except that it should be done monthly when a patient is on a dosage of more than 15 mg per kilogram per day. Snellen eye charts are recommended for testing of visual acuity. Studies have shown that there are definite fluctuations of one or two lines of the Snellen chart in the visual acuity of many tuberculous patients not receiving ethambutol.
The following table may be useful in interpreting possible changes in visual acuity attributable to ethambutol.
Initial
Snellen
Reading
Reading Indicating
Significant Decrease
Significant
Number of Lines
Decrease
Number of Points
20/13
20/25
3
12
20/15
20/25
2
10
20/20
20/30
2
10
20/25
20/40
2
15
20/30
20/50
2
20
20/40
20/70
2
30
20/50
20/70
1
20
In general, changes in visual acuity less than those indicated under "Significant Number of Lines" and "Decrease Number of Points", may be due to chance variation, limitations of the testing method or physiologic variability. Conversely, changes in visual acuity equaling or exceeding those under "Significant Number of Lines" and "Decrease Number of Points" indicate the need for retesting and careful evaluation of the patient’s visual status. If careful evaluation confirms the magnitude of visual change and fails to reveal another cause, ethambutol should be discontinued and the patient reevaluated at frequent intervals. Progressive decreases in visual acuity during therapy must be considered to be due to ethambutol.
If corrective glasses are used prior to treatment, these must be worn during visual acuity testing. During 1 to 2 years of therapy, a refractive error may develop which must be corrected in order to obtain accurate test results. Testing the visual acuity through a pinhole eliminates refractive error. Patients developing visual abnormality during ethambutol treatment may show subjective visual symptoms before, or simultaneously with, the demonstration of decreases in visual acuity, and all patients receiving ethambutol should be questioned periodically about blurred vision and other subjective eye symptoms.
Recovery of visual acuity generally occurs over a period of weeks to months after the drug has been discontinued. Some patients have received ethambutol hydrochloride again after such recovery without recurrence of loss of visual acuity. Other adverse reactions reported include: hypersensitivity, anaphylactic/anaphylactoid reaction, dermatitis, erythema multiforme, pruritus, and joint pain; anorexia, nausea, vomiting, gastrointestinal upset, and abdominal pain; fever, malaise, headache, and dizziness; mental confusion, disorientation, and possible hallucinations; thrombocytopenia, leucopenia, and neutropenia. Numbness and tingling of the extremities due to peripheral neuritis have been reported. Elevated serum uric acid levels occur and precipitation of acute gout has been reported. Pulmonary infiltrates, with or without eosinophilia, also have been reported during ethambutol hydrochloride therapy. Liver toxicities, including fatalities, have been reported. (See WARNINGS). Since ethambutol hydrochloride is recommended for therapy in conjunction with one or more other antituberculous drugs, these changes may be related to the concurrent therapy. Hypersensitivity syndrome consisting of cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, and one or more of the following: hepatitis, pneumonitis, nephritis, myocarditis, pericarditis. Fever and lymphadenopathy may be present.
-
DOSAGE AND ADMINISTRATION
Ethambutol hydrochloride should not be used alone, in initial treatment or in retreatment. Ethambutol hydrochloride should be administered on a once every 24-hour basis only. Absorption is not significantly altered by administration with food. Therapy, in general, should be continued until bacteriological conversion has become permanent and maximal clinical improvement has occurred.
Ethambutol hydrochloride is not recommended for use in pediatric patients under thirteen years of age since safe conditions for use have not been established.
Initial Treatment
In patients who have not received previous antituberculous therapy, administer ethambutol hydrochloride 15 mg/ kg (7 mg/lb) of body weight, as a single oral dose once every 24 hours. In the more recent studies, isoniazid has been administered concurrently in a single, daily, oral dose.
Retreatment
In patients who have received previous antituberculous therapy, administer ethambutol hydrochloride 25 mg/kg (11 mg/lb) of body weight, as a single oral dose once every 24 hours. Concurrently administer at least one other antituberculous drug to which the organisms have been demonstrated to be susceptible by appropriate in-vitro tests. Suitable drugs usually consist of those not previously used in the treatment of the patient. After 60 days of ethambutol hydrochloride administration, decrease the dose to 15 mg/kg (7 mg/lb) of body weight, and administer as a single oral dose once every 24 hours.
During the period when a patient is on a daily dose of 25 mg/kg, monthly eye examinations are advised.
See Table for easy selection of proper weight-dose tablet(s).
Weight-Dose Table 15 mg/kg (7 mg/lb) Schedule
Weight Range
Daily Dose
Pounds
Kilograms
-----------------
In mg
Under 85 lbs.
Under 37 kg
-----------------
500
85 – 94.5
37 – 43
-----------------
600
95 – 109.5
43 – 50
-----------------
700
110 – 124.5
50 – 57
-----------------
800
125 – 139.5
57 – 64
-----------------
900
140 – 154.5
64 – 71
-----------------
1000
155 – 169.5
71 – 79
-----------------
1100
170 – 184.5
79 – 84
-----------------
1200
185 – 199.5
84 – 90
-----------------
1300
200 – 214.5
90 – 97
-----------------
1400
215 and Over
Over 97
-----------------
1500
25 mg/kg (11 mg/lb) Schedule
Under 85 lbs.
Under 38 kg
-----------------
900
85 – 92.5
38 – 42
-----------------
1000
93 – 101.5
42 – 45.5
-----------------
1100
102 – 109.5
45.5 – 50
-----------------
1200
110 – 118.5
50 – 54
-----------------
1300
119 – 128.5
54 – 58
-----------------
1400
129 – 136.5
58 – 62
-----------------
1500
137 – 146.5
62 – 67
-----------------
1600
147 – 155.5
67 – 71
-----------------
1700
156 – 164.5
71 – 75
-----------------
1800
165 – 173.5
75 – 79
-----------------
1900
174 – 182.5
79 – 83
-----------------
2000
183 – 191.5
83 – 87
-----------------
2100
192 – 199.5
87 – 91
-----------------
2200
200 – 209.5
91 – 95
-----------------
2300
210 – 218.5
95 – 99
-----------------
2400
219 and Over
Over 99
-----------------
2500
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- Ethambutol 400mg Tablets
-
INGREDIENTS AND APPEARANCE
ETHAMBUTOL HYDROCHLORIDE
ethambutol hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53002-6580(NDC:68180-281) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHAMBUTOL HYDROCHLORIDE (UNII: QE4VW5FO07) (ETHAMBUTOL - UNII:8G167061QZ) ETHAMBUTOL HYDROCHLORIDE 400 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off-white) Score 2 pieces Shape ROUND (Biconvex) Size 12mm Flavor Imprint Code L;U;C32 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53002-6580-0 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078939 07/04/2009 Labeler - RPK Pharmaceuticals, Inc. (147096275) Establishment Name Address ID/FEI Business Operations RPK Pharmaceuticals, Inc. 147096275 RELABEL(53002-6580) , REPACK(53002-6580)