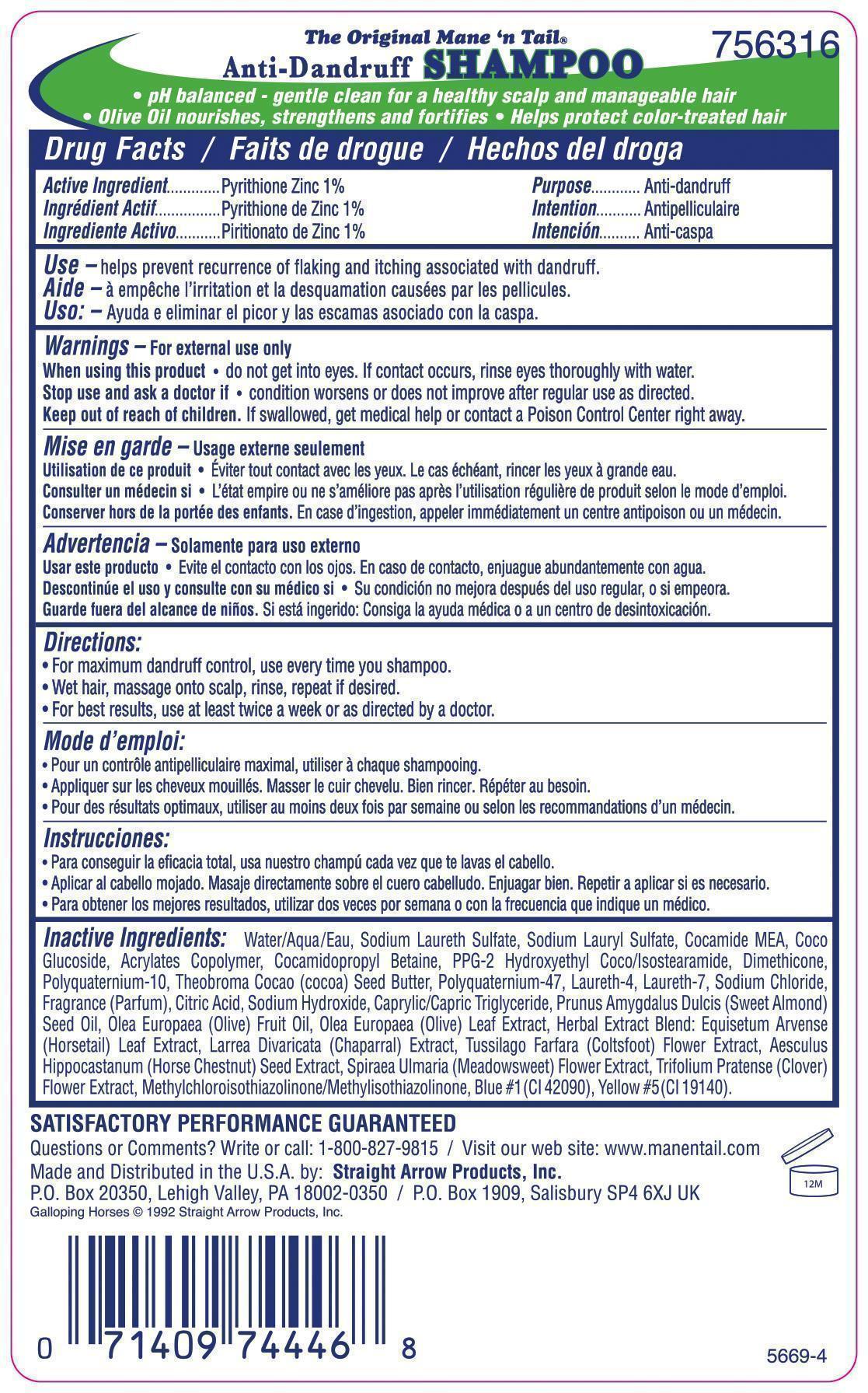
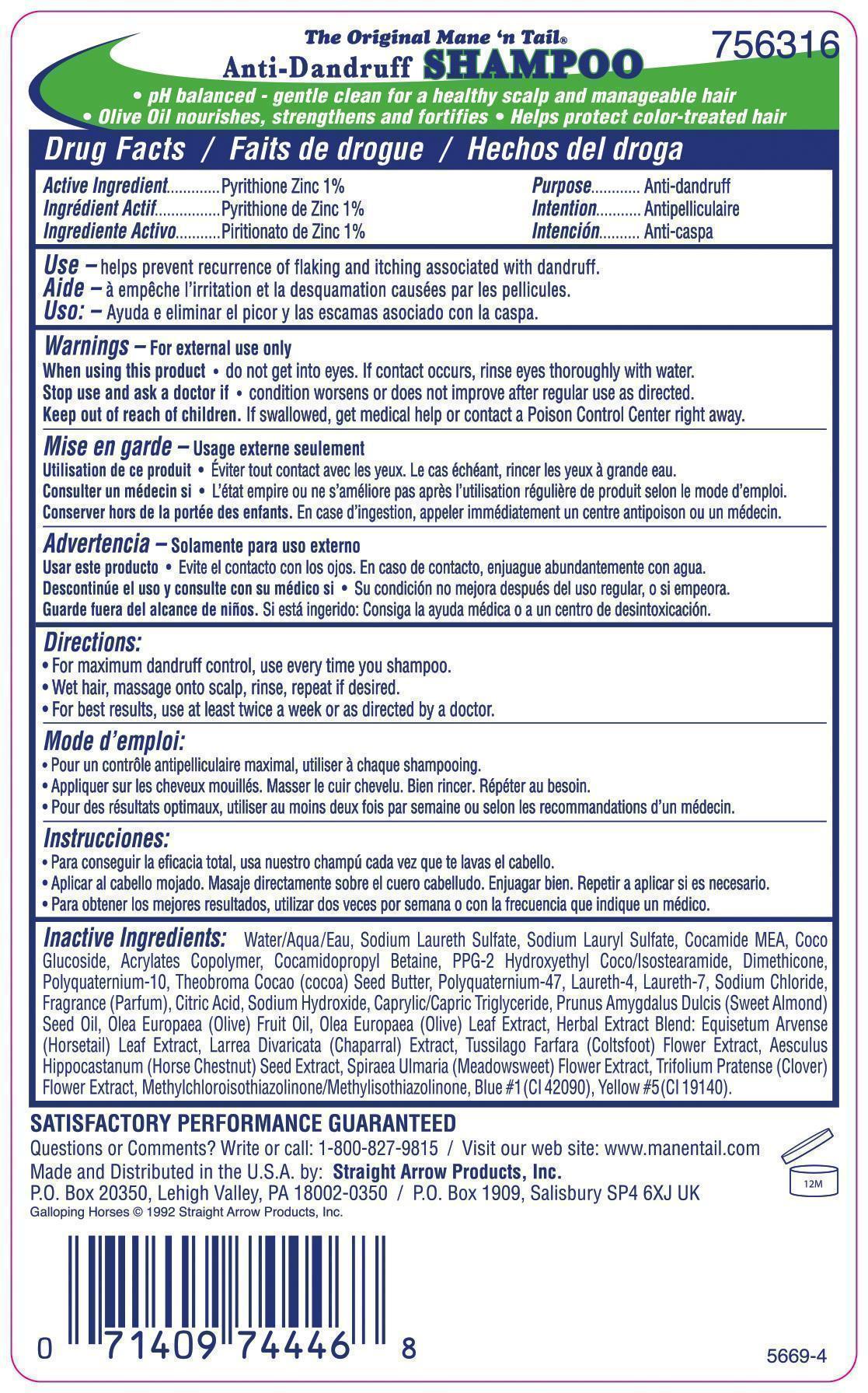
Label: THE ORIGINAL MANE N TAIL DAILY CONTROL ANTI DANDRUFF- pyrithione zinc shampoo
-
Contains inactivated NDC Code(s)
NDC Code(s): 62001-0331-1, 62001-0331-2, 62001-0331-3 - Packager: Straight Arrow Products Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 10, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients: Aqua (Water), Sodium Laureth Sulfate, Sodium Lauryl Sulfate, Cocamide MEA, Coco Glucoside, Acrylates Copolymer, Cocamidopropyl Betaine, PPG-2 Hydroxyethyl Coco/Isostearamide, Dimethicone, Polyquaternium-10, Theobroma Cocao (cocoa) Seed Butter, Polyquaternium-47, Laureth-4, Laureth-7, Sodium Chloride, Fragrance (Parfum), Citric Acid, Sodium Hydroxide, Caprylic/Capric Triglyceride, Prunus Amygdalus Dulcis (Sweet Almond) Seed Oil, Olea Europaea (Olive) Fruit Oil, Olea Europaea (Olive) Leaf Extract, Herbal Extract Blend: Equisetum Arvense (Horsetail) Leaf Extract, Larrea Divaricata (Chaparral) Extract, Tussilago Farfara (Coltsfoot) Flower Extract, Aesculus Hippocastanum (Horse Chestnut) Seed Extract, Spiraea Ulmaria (Meadowsweet) Flower Extract, Trifolium Pratense (Clover) Flower Extract, Methylchloroisothiazolinone/ Methylisothiazolinone, Blue #1 (CI 42090), Yellow #5 (CI 19140).
- The Original Mane n Tail Daily Control Anti Dandruff Shampoo
-
INGREDIENTS AND APPEARANCE
THE ORIGINAL MANE N TAIL DAILY CONTROL ANTI DANDRUFF
pyrithione zinc shampooProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62001-0331 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRITHIONE ZINC (UNII: R953O2RHZ5) (PYRITHIONE ZINC - UNII:R953O2RHZ5) PYRITHIONE ZINC .01 g in .01 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) SODIUM LAURYL SULFATE (UNII: 368GB5141J) COCO MONOETHANOLAMIDE (UNII: C80684146D) COCO GLUCOSIDE (UNII: ICS790225B) CARBOMER INTERPOLYMER TYPE A (55000 MPA.S) (UNII: 59TL3WG5CO) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) PPG-2 HYDROXYETHYL COCAMIDE (UNII: 34N07GUJ3X) DIMETHICONE (UNII: 92RU3N3Y1O) POLYQUATERNIUM-10 (400 CPS AT 2%) (UNII: HB1401PQFS) COCOA BUTTER (UNII: 512OYT1CRR) POLYQUATERNIUM-47 (METHACRYLAMIDOPROPYLTRIMETHYLAMMONIUM CHLORIDE-CO-METHYL ACRYLATE-CO-ACRYLIC ACID 45:10:45; 1200000 MW) (UNII: F11YNO8FDQ) LAURETH-4 (UNII: 6HQ855798J) LAURETH-7 (UNII: Z95S6G8201) SODIUM CHLORIDE (UNII: 451W47IQ8X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM HYDROXIDE (UNII: 55X04QC32I) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) ALMOND OIL (UNII: 66YXD4DKO9) OLIVE OIL (UNII: 6UYK2W1W1E) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) EQUISETUM ARVENSE TOP (UNII: 1DP6Y6B65Z) LARREA DIVARICATA LEAF (UNII: I15RZ48987) TUSSILAGO FARFARA (UNII: 0JXZ63016V) HORSE CHESTNUT (UNII: 3C18L6RJAZ) FILIPENDULA ULMARIA FLOWER (UNII: 06L18L32G6) TRIFOLIUM PRATENSE FLOWER (UNII: 4JS0838828) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color green Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62001-0331-1 473 g in 1 BOTTLE, PLASTIC 2 NDC:62001-0331-2 10.5 g in 1 POUCH 3 NDC:62001-0331-3 7.1 g in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 04/20/2008 Labeler - Straight Arrow Products Inc (061580593) Registrant - Straight Arrow Products Inc (061580593) Establishment Name Address ID/FEI Business Operations Straight Arrow Products Inc 061580593 manufacture(62001-0331) , label(62001-0331) , pack(62001-0331)