Label: CAPMIST DM- dextromethorphan hydrobromide, guaifenesin and pseudoephedrine hydrochloride tablet
- NDC Code(s): 29978-601-30, 29978-601-90
- Packager: Capital Pharmaceutical, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
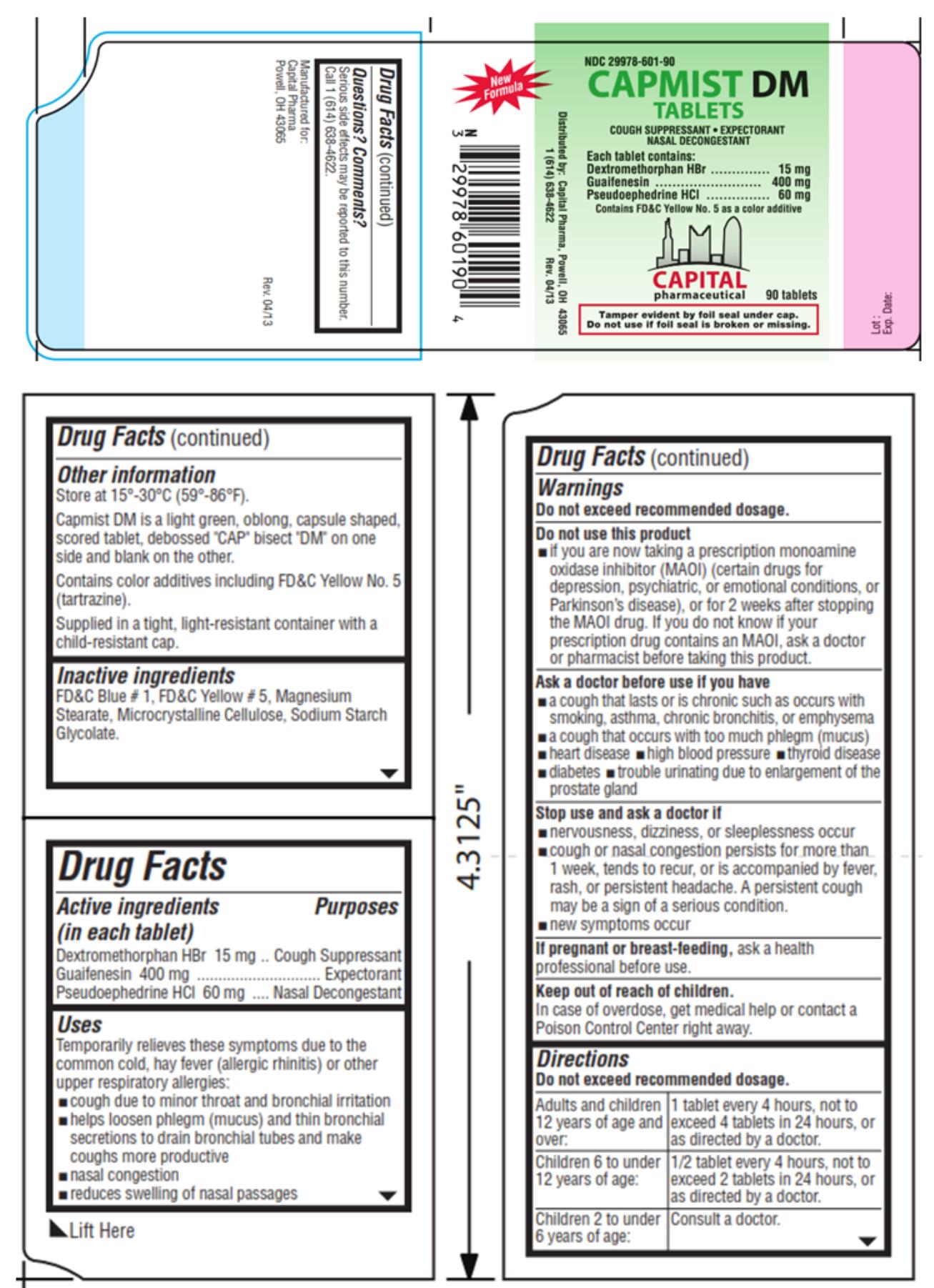
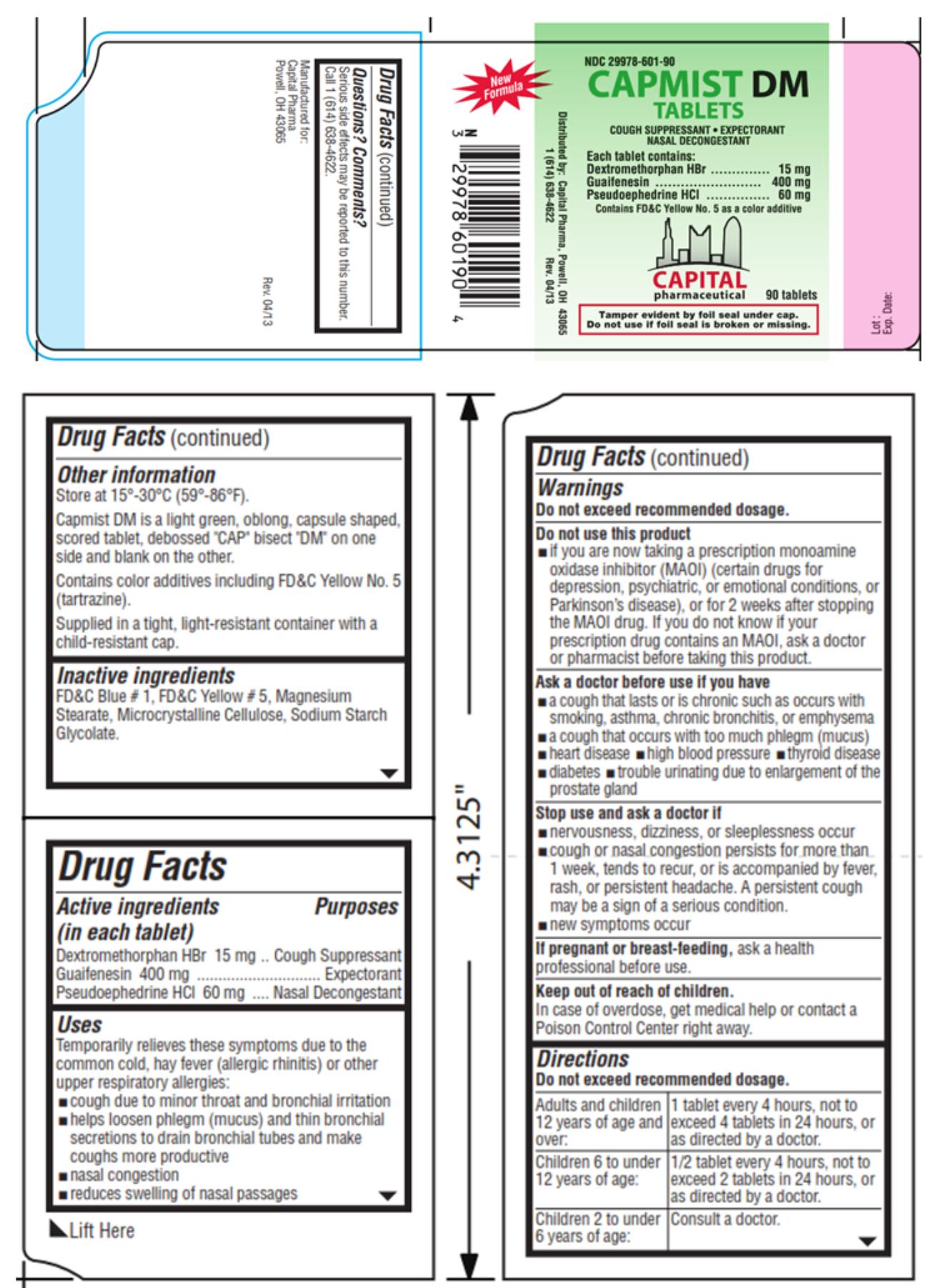
- Active ingredients
- Purpose
- Active ingredients
- Purpose
- Active ingredients
- Purpose
-
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes and make coughs more productive
- nasal congestion
- reduces swelling of nasal passages
- cough due to minor throat and bronchial irritation
-
Warnings
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- a cough that occurs with too much phlegm (mucus)
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to enlargement of the prostate gland
-
Directions
Do not exceed recommended dosage. Adults and children 12 years of age and over: 1 tablet every 4 hours, not to exceed 4 tablets in 24 hours, or as directed by a doctor. Children 6 to under 12 years of age: 1/2 tablet every 4 hours, not to exceed 2 tablets in 24 hours, or as directed by a doctor. Children 2 to under 6 years of age: Consult a doctor. -
Other information
Store at 15°-30°C (59°-86°F).
Capmist DM is a light green, oblong, capsule shaped, scored tablet, debossed “CAP” bisect “DM” on one side and blank on the other.
Contains color additives including FD&C Yellow No. 5 (tartrazine).
Supplied in a tight, light-resistant container with a child-resistant cap.
- Inactive ingredients
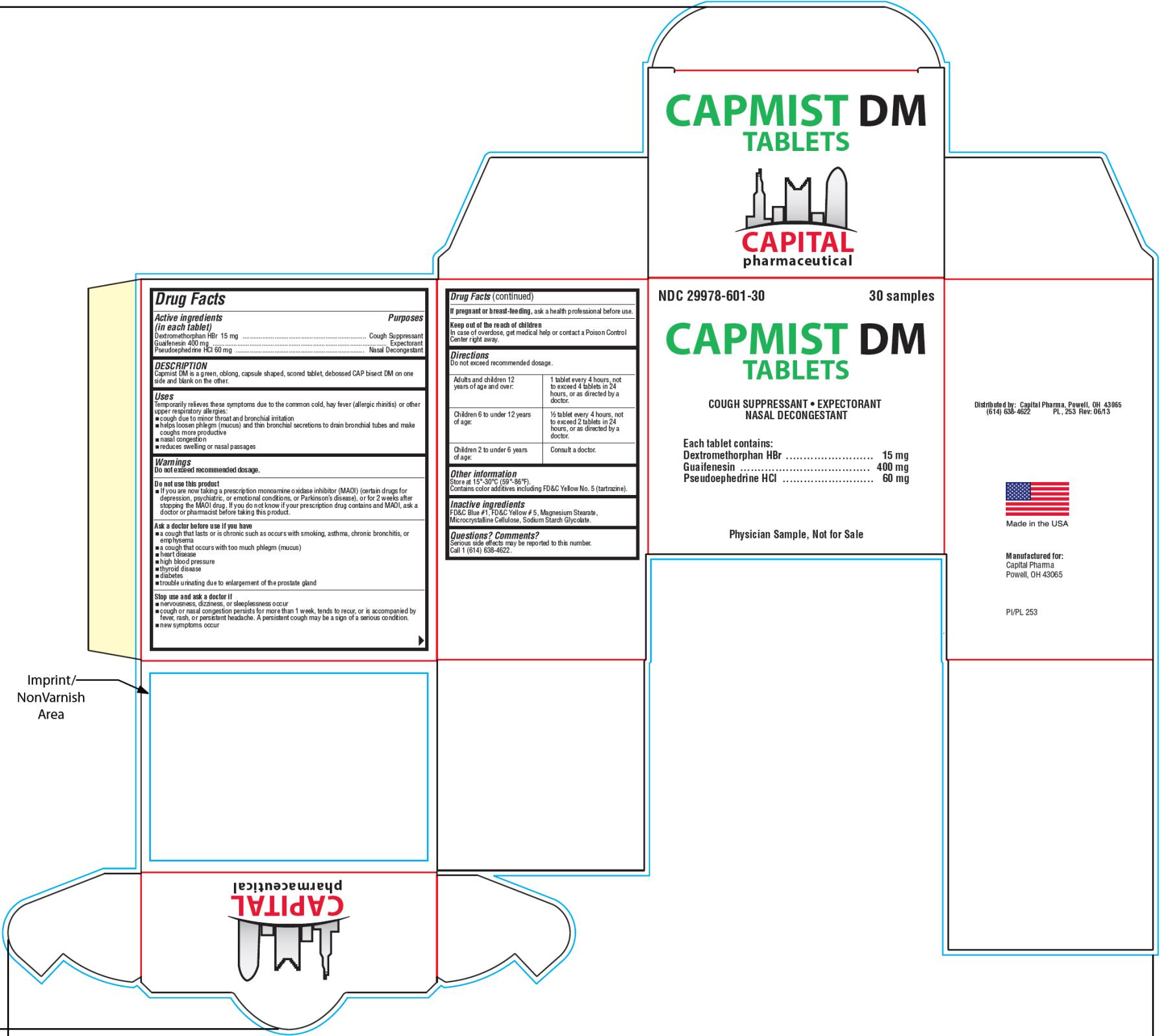
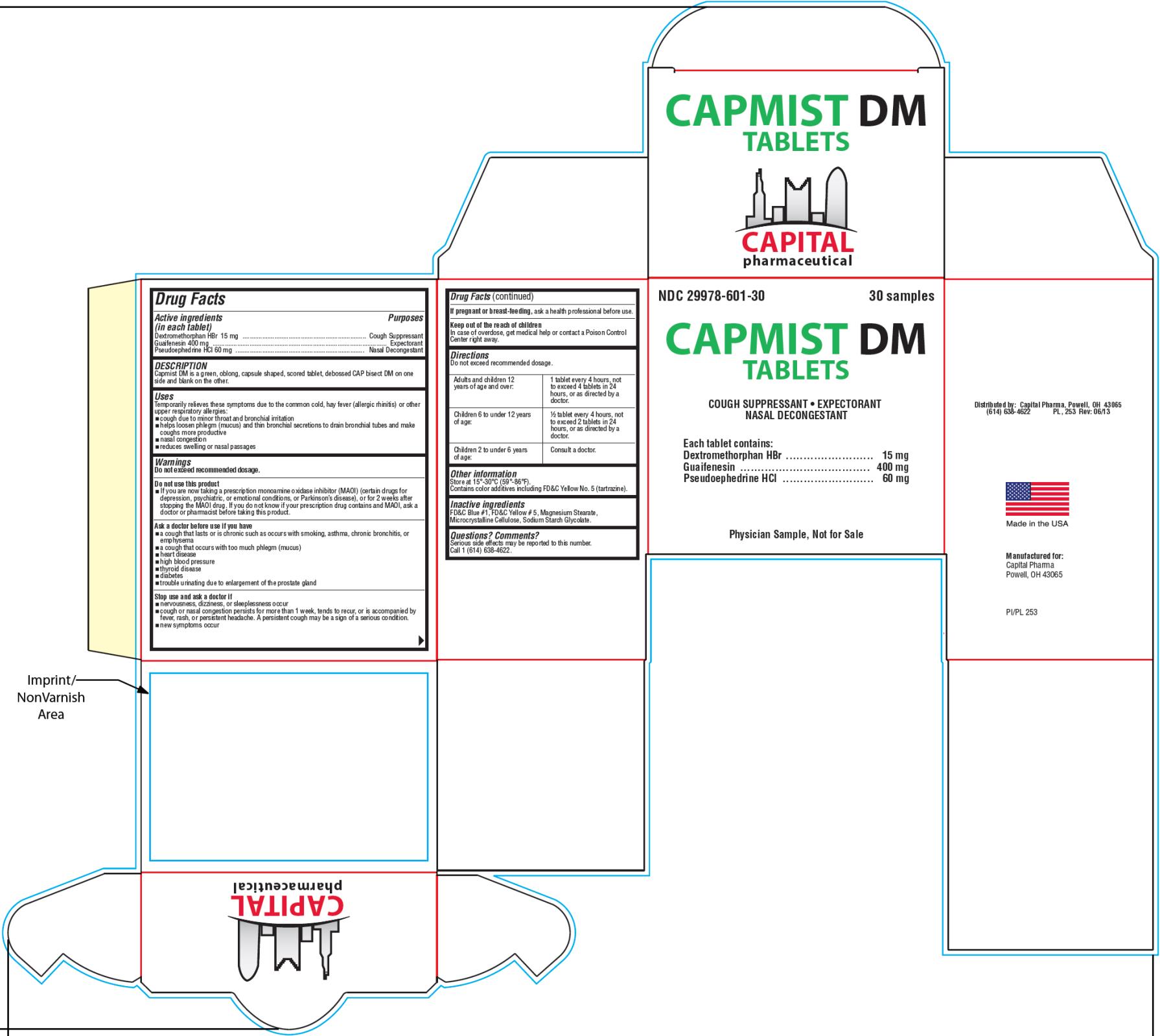
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CAPMIST DM
dextromethorphan hydrobromide, guaifenesin and pseudoephedrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:29978-601 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 15 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color green (light green) Score 2 pieces Shape CAPSULE (oblong) Size 19mm Flavor Imprint Code CAP;DM Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:29978-601-30 2 in 1 BOX 06/20/2013 1 15 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:29978-601-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/20/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/20/2013 Labeler - Capital Pharmaceutical, LLC (831545541)