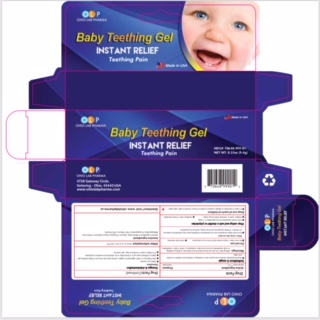
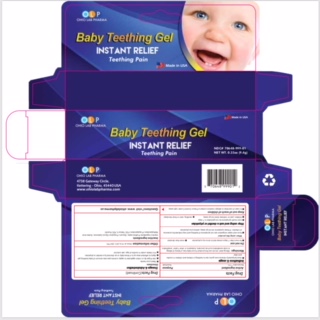
Label: OLP BABY TEETHING- benzocaine gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 70648-999-01 - Packager: Ohio Lab Pharma
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 1, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Uses
- temporarily relieves sore gums due to teething in infants and children 4 months of age and older
Warnings
- Allergy alert: do not use this product if you have a history of allergy to local anesthetics such as
procaine, butacaine, benzocaine or other “caine” anesthetics
- Do not use more than directed for more than 7 days unless told to do so by a dentist or doctor.
- When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms do not go away, advise your dentist or doctor.
- Stop use and ask a doctor if sore mouth symptoms do not get better in 7 days. swelling, rash or fever develops, irritation, pain or redness persists or worsens
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Purpose
- Active Ingredient
- Other information
- Directions
- Inactive ingredients
- when using this product
- stop using and ask a dentist or physician
- keep out of reach of children
- questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OLP BABY TEETHING
benzocaine gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70648-999 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 7.5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBIC ACID (UNII: X045WJ989B) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) CARBOMER 940 (UNII: 4Q93RCW27E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70648-999-01 1 in 1 CARTON 12/06/2017 1 9.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 12/06/2017 Labeler - Ohio Lab Pharma (080215854) Establishment Name Address ID/FEI Business Operations Ohio Lab Pharma 080215854 manufacture(70648-999)