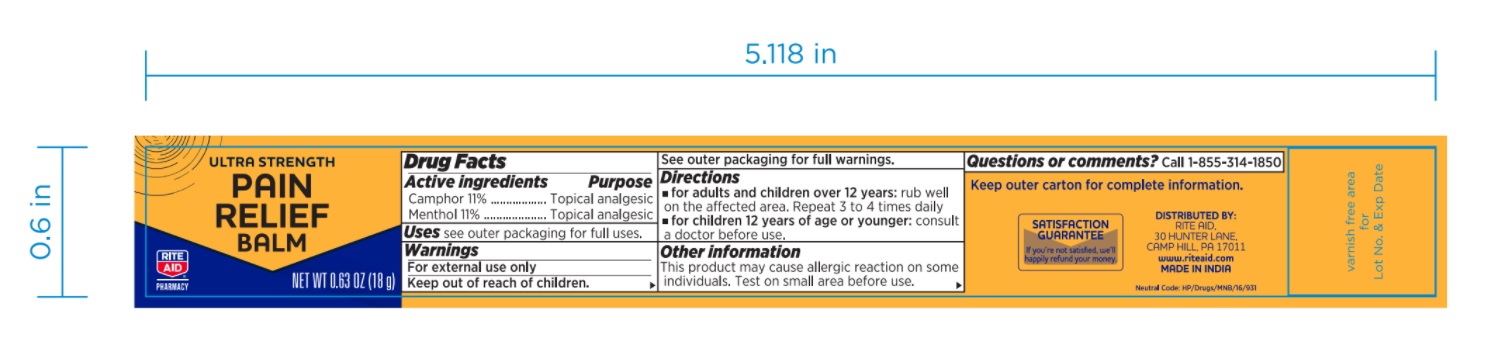
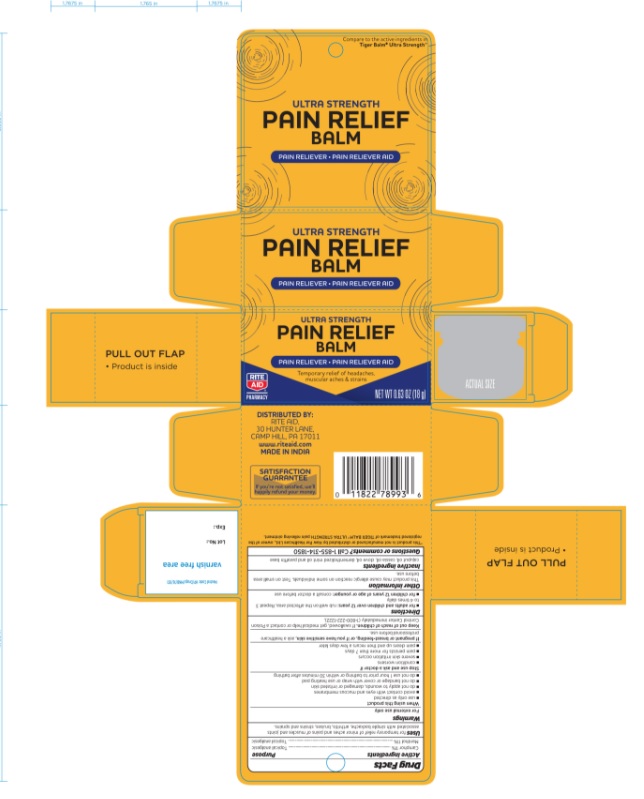
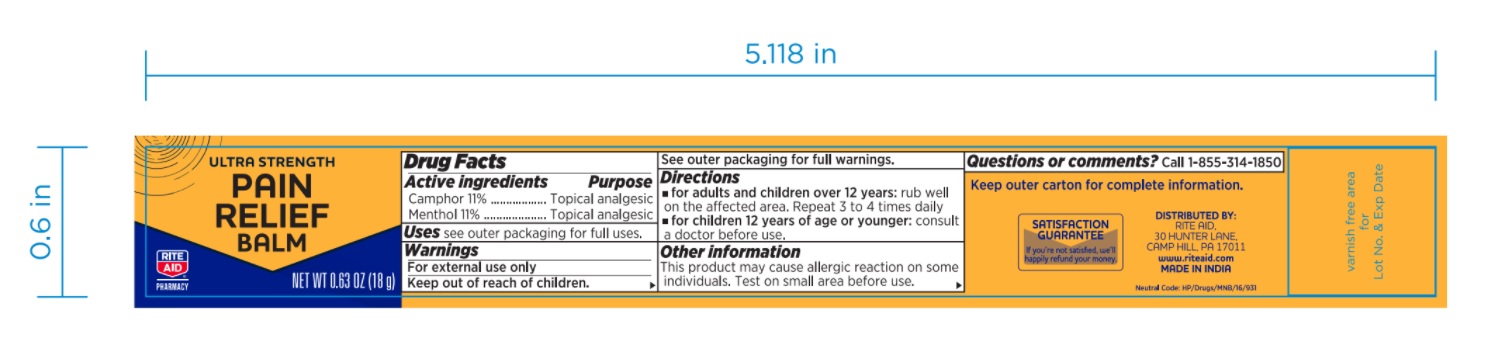
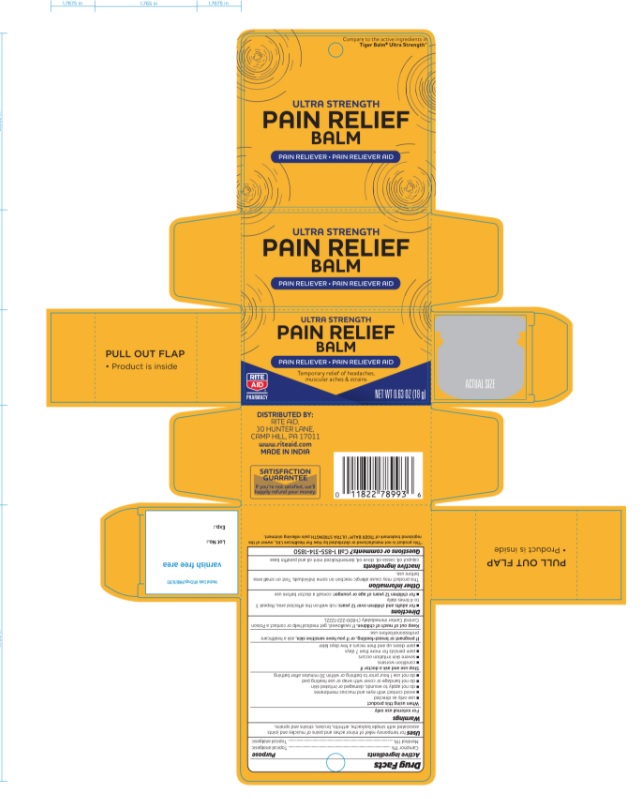
Label: ULTRA STRENGTH PAIN RELIEF BALM ULTRA STRENGTH (camphor- synthetic and menthol ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 76168-331-18 - Packager: Velocity Pharma LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
For External Use Only
When using this product
- Use only as directed
- Avoid contact with eyes and mucous membranes
- Do not apply to wounds, damaged or irritated skin
- Do not bandage or cover with wrap or use heating pad
- Do not use 1 hour prior to bathing or within 30 minutes after bathing.
Stop use and ask a doctor if
- Condition worsens
- Severe skin irritation occurs
- Pain persists for more than 7 days
- Pain clears up and then recurs a few days later.
- Directions
- Other information
- Inactive Ingredients
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ULTRA STRENGTH PAIN RELIEF BALM ULTRA STRENGTH
camphor (synthetic) and menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76168-331 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 110 mg in 1 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 110 mg in 1 g Inactive Ingredients Ingredient Name Strength CAJUPUT OIL (UNII: J3TO6BUQ37) PADANG CASSIA OIL (UNII: 0E15252LIW) CLOVE OIL (UNII: 578389D6D0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76168-331-18 1 in 1 PACKAGE 09/10/2020 1 18 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/10/2020 Labeler - Velocity Pharma LLC (962198409) Registrant - Velocity Pharma LLC (962198409)