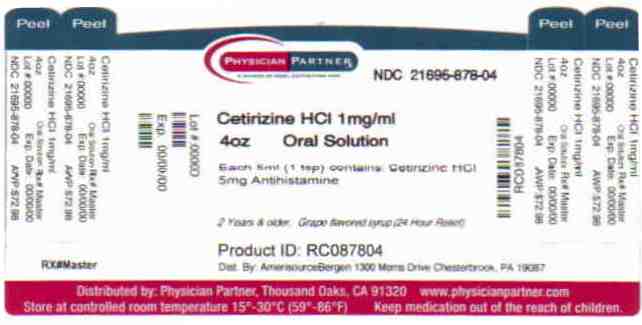
Label: CETIRIZINE liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 21695-878-04 - Packager: Rebel Distributors Corp
- This is a repackaged label.
- Source NDC Code(s): 24385-188
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 15, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Keep Out of Reach of Children
- PURPOSE
- Uses
-
Warnings
Do not use if you have ever had a allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
Ask a doctor or pharmacist before use if you are taking tranquilizers or sedatives.
When using this product
° drowsiness may occur ° avoid alcoholic drinks ° alcohol, sedatives and tranquilizers may increase drowsiness ° be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
If pregnant or breast-feeding:
° if breast-feeding: not recommended
° if pregnant: ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
-
Directions
Use only with enclosed dosing cup:
Adults and children 6 years and over: 1 teaspoonful (5ml) or 2 teaspoonfuls (10ml) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10ml) in 24 hours.
Adults 65 years and over: 1 teaspoonful (5mo) once daily; do not take more than 1 teaspoonful (5ml) in 24 hours.
Children 2 to under 6 years of age:
1/2 teaspoonful (2.5ml) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5mo) once daily or 1/2 teaspoonful (2.5ml) every 12 hours. Do not give more than 1 teaspoonful (5ml) in 24 hours.
Children under 2 years of age: ask a doctor
Consumers with liver or kidney disease: ask a doctor
- Other Information
- Inactive ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CETIRIZINE
cetirizine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21695-878(NDC:24385-188) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM ACETATE (UNII: 4550K0SC9B) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21695-878-04 120 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078398 06/17/2008 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK