Label: CLOROX HEALTHCARE GBG ALOEGEL- alcohol cloth
-
NDC Code(s):
69540-0037-1,
69540-0037-2,
69540-0037-3,
69540-0037-4, view more69540-0037-5, 69540-0037-6
- Packager: Brand Buzz LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other Information
- Ingredients
- Questions of Comments?
-
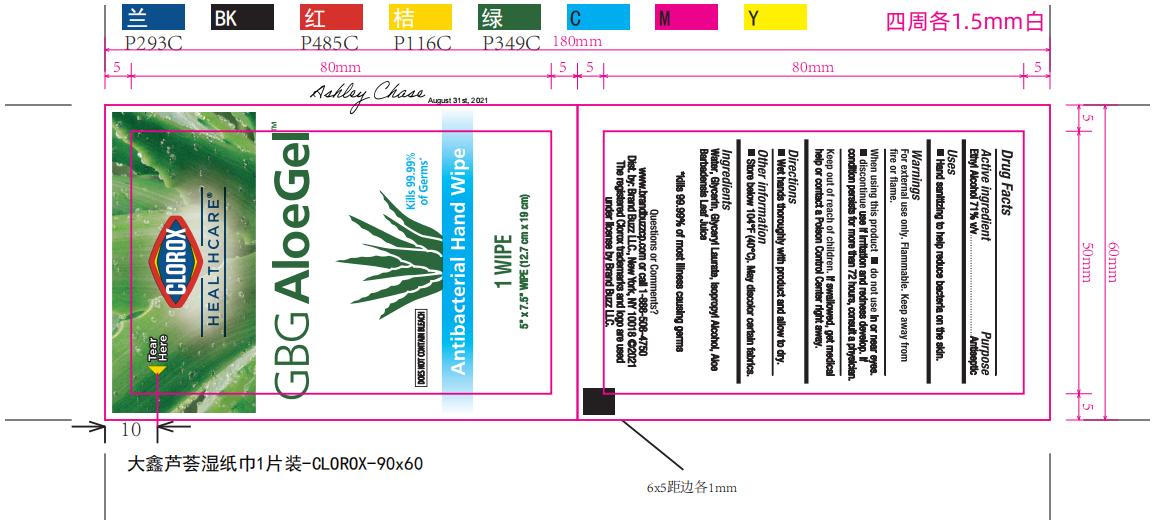
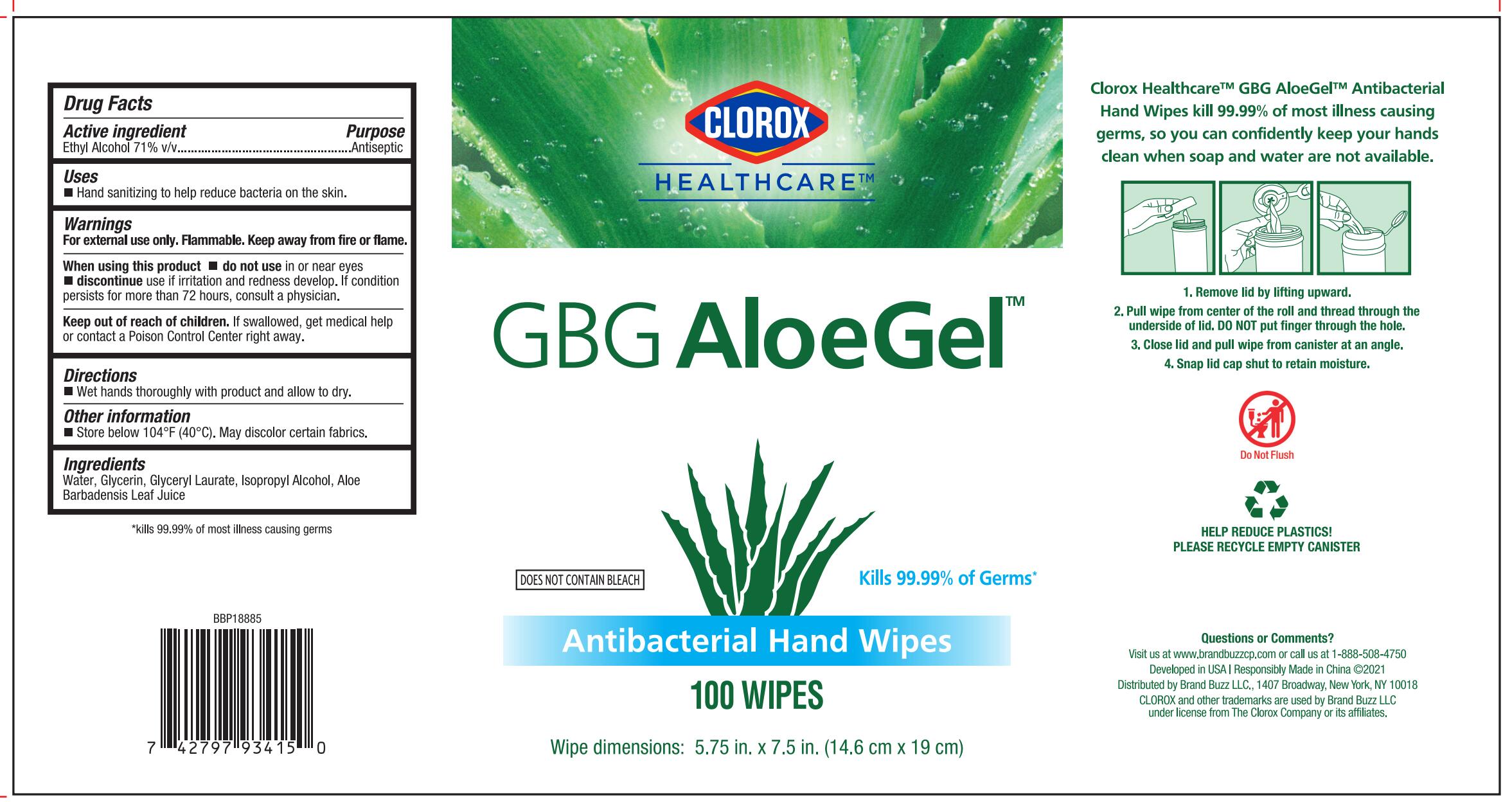
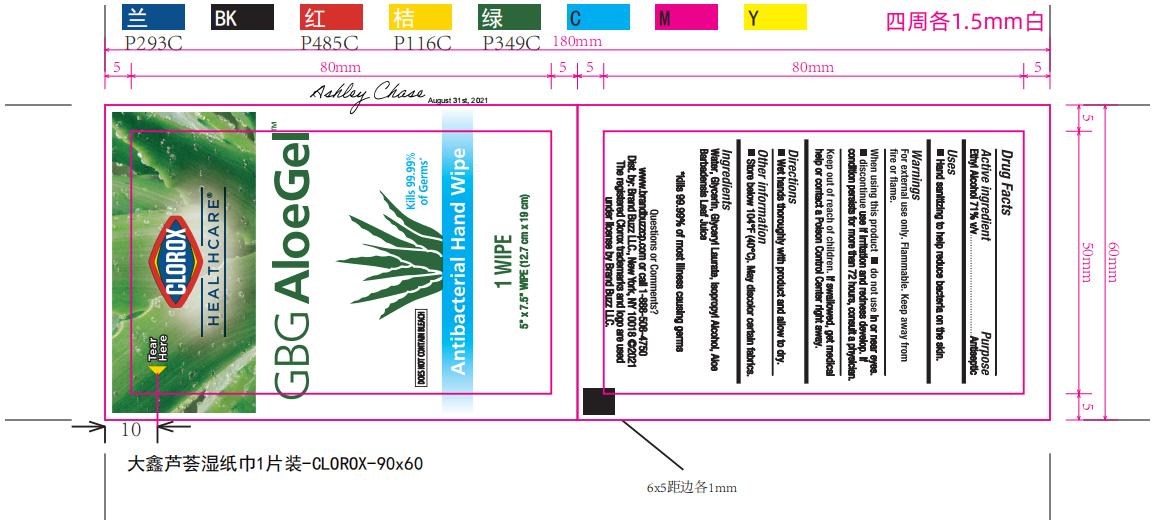
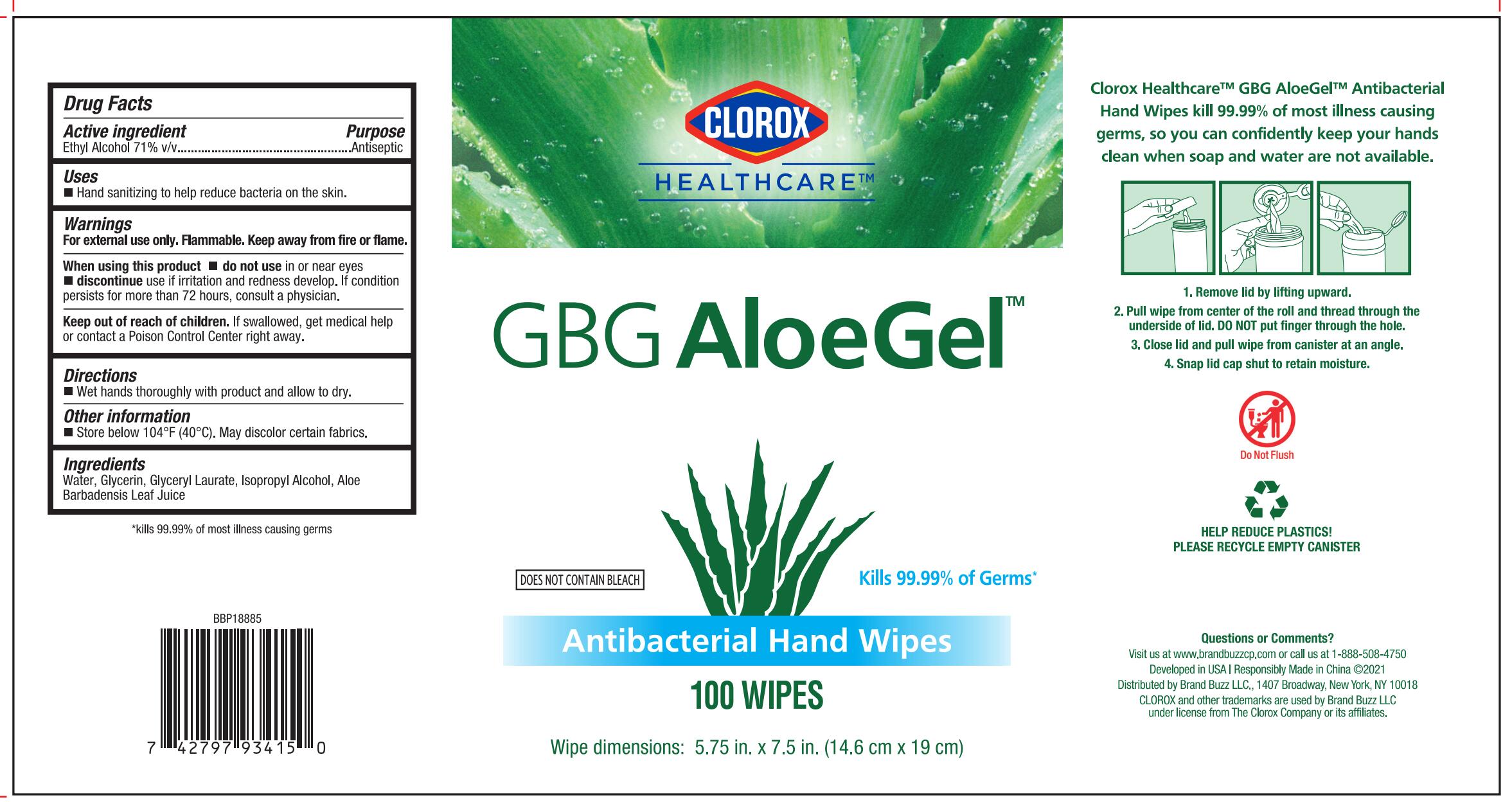
PRINCIPAL DISPLAY PANEL
CLOROX HEALTHCARE™
GBG AloeGel™
Kills 99.99% Germs*
DOES NOT CONTAIN BLEACH
Antibacterial Hand Wipes
Clorox Healthcare™ GBG AloeGel™ Antibacterial Hand Wipes kill 99.99% of most illness causing germs, so you can confidently keep your hands clean when soap and water are not available
Wipe dimensions: 5.75in. x 7.5in. (14.6 cm x 19 cm)
- Remove lid by lifting upward.
- Pull wipe from center of the roll and thread through the underside of lid. DO NOT put finger through the hole.
- Close lid and pull wipe from canister at an angle.
- Snap lid cap shut to retain moisture.
Developed in USA | Responsibly Made in China ©2021
Distributed by Brand Buzz LLC., 1407 Broadway, New York, NY 10018
CLOROX and other trademarks are used by Brand Buzz LLC under license from The Clorox Company or its affiliates.
*kills 99.99% of most illness causing germs
1x100 Wipes

20 Wipes

40 Wipes

100 Wipes
270 Wipes

CLOROX™
Hand Sanitizing Wipe
Kills 99.99% Germs*
Alcohol-Based
Bleach-Free
For use on hands only
1 Wipe: 5in. x 7.5in. (12.7cm x 19cm)
*kills 99.99% of most illness causing germs
Dist. by: Brand Buzz LLC., 1407 Broadway, New York, NY 10018
The registered Clorox trademarks and logo are used under license by Brand Buzz LLC.
1x40 Wipes

-
INGREDIENTS AND APPEARANCE
CLOROX HEALTHCARE GBG ALOEGEL
alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69540-0037 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.71 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL LAURATE (UNII: Y98611C087) ALOE VERA LEAF (UNII: ZY81Z83H0X) ISOPROPYL ALCOHOL (UNII: ND2M416302) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69540-0037-2 20 in 1 POUCH 10/04/2021 1 0.71 mL in 1 PATCH; Type 0: Not a Combination Product 2 NDC:69540-0037-3 100 in 1 CANISTER 10/04/2021 2 0.71 mL in 1 PATCH; Type 0: Not a Combination Product 3 NDC:69540-0037-4 270 in 1 CANISTER 10/04/2021 3 0.71 mL in 1 PATCH; Type 0: Not a Combination Product 4 NDC:69540-0037-5 40 in 1 CANISTER 10/04/2021 4 0.71 mL in 1 PATCH; Type 0: Not a Combination Product 5 NDC:69540-0037-1 100 in 1 BOX 10/04/2021 5 0.71 mL in 1 PACKET; Type 0: Not a Combination Product 6 NDC:69540-0037-6 40 in 1 BOX 10/04/2021 6 0.71 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 08/24/2021 Labeler - Brand Buzz LLC (079266204)