Label: GLYCERIN suppository
-
Contains inactivated NDC Code(s)
NDC Code(s): 50730-1091-0 - Packager: H and P Industries, Inc. dba Triad Group
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USE
-
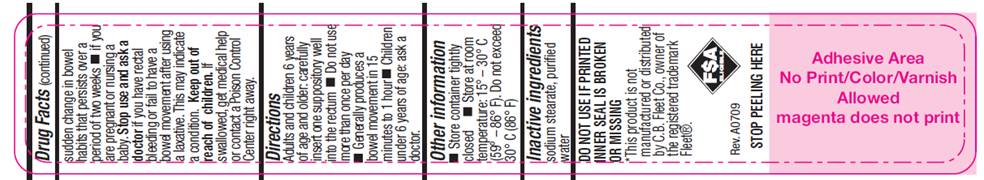
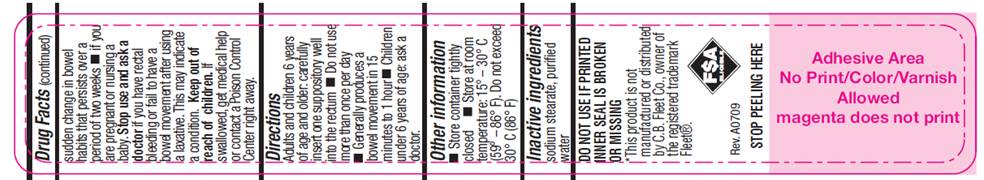
WARNINGS
For rectal use only. May cause rectal discomfort or a burning sensation.
Do not use
- more than one per day
- for a period of longer than one week unless directed by a doctor
- laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor
- if seal under product lid is damaged, missing or broken.
Ask a doctor before use
- if you have noticed a sudden change in bowel habits that persist over a period of two weeks
- if you are pregnant or nursing a baby
- DIRECTIONS
- SPL UNCLASSIFIED SECTION
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS
-
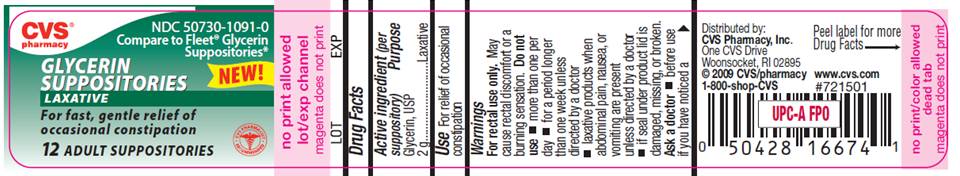
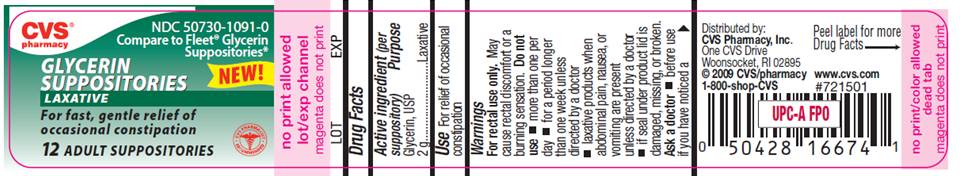
PACKAGE LABEL
CVS pharmacy
NDC 50730-1091-0
Compare to Fleet Glycerin Suppositories
NEW!
GLYCERIN
SUPPOSITORIES
LAXATIVE
For fast, gentle relief of
occasional constipation
12 ADULT SUPPOSITORIES
Distributed by CVS Pharmacy, Inc.
One CVS Drive
Woonsocket, RI 02895
©CVS/pharmacy
DO NOT USE IF PRINTED
1-800-shop-CVS
www.cvs.com
#721501
INNER SEAL IS BROKEN
OR MISSING
This product is not
manufactured or distributed
by C.B.Fleet co., owner of
the registered trademark
Fleet®.
-
INGREDIENTS AND APPEARANCE
GLYCERIN
glycerin suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50730-1091 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength glycerin (UNII: PDC6A3C0OX) (glycerin - UNII:PDC6A3C0OX) glycerin 2 g Inactive Ingredients Ingredient Name Strength sodium stearate (UNII: QU7E2XA9TG) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50730-1091-0 12 in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part334 07/01/2009 Labeler - H and P Industries, Inc. dba Triad Group (050259597) Registrant - H and P Industries, Inc. dba Triad Group (050259597) Establishment Name Address ID/FEI Business Operations H and P Industries, Inc. dba Triad Group 050259597 manufacture