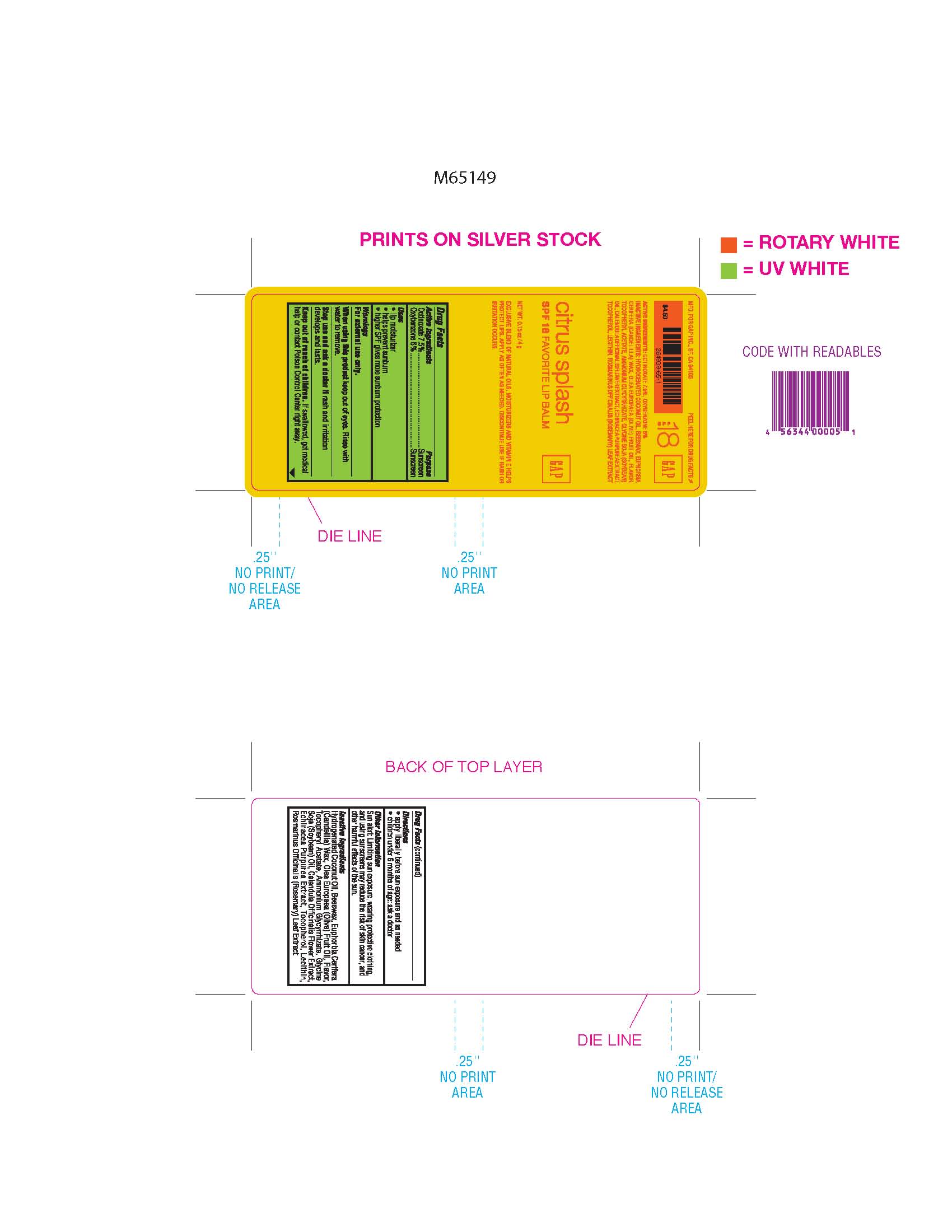
Label: GAP FAVORITE LIP BALM CITRUS SPLASH SPF 18- octinoxate and oxybenzone stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 51514-0212-1 - Packager: Autumn Harp, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 16, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Warning
- When using this product
- Stop use
- Keep out of reach of children
- Directions
- Sun Alert
-
Inactive Ingredients
Inactive Ingredients: Hydrogenated Coconut Oil, Beeswax, Euphorbia Cerifera (Candelilla) Wax, Olea Europaea (Olive) Fruit Oil, Flavor, Tocopheryl Acetate, Ammonium Glycyrrhizate, Glycine Soja (Soybean) Oil, Calendula Officinalis Flower Extract, Echinacea Purpurea Extract, Tocopherol, Lecithin, Rosmarinus Officinalis (Rosemary) Leaf Extract.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GAP FAVORITE LIP BALM CITRUS SPLASH SPF 18
octinoxate and oxybenzone stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51514-0212 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) OLIVE OIL (UNII: 6UYK2W1W1E) YELLOW WAX (UNII: 2ZA36H0S2V) CANDELILLA WAX (UNII: WL0328HX19) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) TOCOPHEROL (UNII: R0ZB2556P8) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ROSEMARY (UNII: IJ67X351P9) ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) AMMONIUM GLYCYRRHIZATE (UNII: 3VRD35U26C) Product Characteristics Color Score Shape Size Flavor GRAPEFRUIT (Grapefruit Lime) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51514-0212-1 4 g in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 11/16/2011 Labeler - Autumn Harp, Inc (064187883) Establishment Name Address ID/FEI Business Operations Autumn Harp, Inc 064187883 manufacture